Cell size control and a cell-intrinsic maturation program in proliferating oligodendrocyte precursor cells
- PMID: 9298991
- PMCID: PMC2132550
- DOI: 10.1083/jcb.138.6.1367
Cell size control and a cell-intrinsic maturation program in proliferating oligodendrocyte precursor cells
Abstract
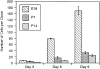
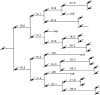
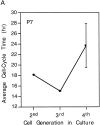
We have used clonal analysis and time-lapse video recording to study the proliferative behavior of purified oligodendrocyte precursor cells isolated from the perinatal rat optic nerve growing in serum-free cultures. First, we show that the cell cycle time of precursor cells decreases with increasing concentrations of PDGF, the main mitogen for these cells, suggesting that PDGF levels may regulate the cell cycle time during development. Second, we show that precursor cells isolated from embryonic day 18 (E18) nerves differ from precursor cells isolated from postnatal day 7 (P7) or P14 nerves in a number of ways: they have a simpler morphology, and they divide faster and longer before they stop dividing and differentiate into postmitotic oligodendrocytes. Third, we show that purified E18 precursor cells proliferating in culture progressively change their properties to resemble postnatal cells, suggesting that progressive maturation is an intrinsic property of the precursors. Finally, we show that precursor cells, especially mature ones, sometimes divide unequally, such that one daughter cell is larger than the other; in each of these cases the larger daughter cell divides well before the smaller one, suggesting that the precursor cells, just like single-celled eucaryotes, have to reach a threshold size before they can divide. These and other findings raise the possibility that such stochastic unequal divisions, rather than the stochastic events occurring in G1 proposed by "transition probability" models, may explain the random variability of cell cycle times seen within clonal cell lines in culture.
Figures









References
-
- Barres B, Raff M. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994;12:935–942. - PubMed
-
- Barres B, Hart I, Coles H, Burne JF, Voyvodic J, Richardson W, Raff M. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. - PubMed
-
- Barres B, Schmid R, Sendtner M, Raff M. Multiple extracellular signals are required for long term oligodendrocyte survival. Development. 1993;118:283–295. - PubMed
-
- Barres B, Lazar M, Raff M. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. - PubMed
-
- Brooks RF, Bennett DC, Smith JA. Mammalian cell cycles need two random transitions. Cell. 1980;19:493–504. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

