Biochemical analysis of the stress protein response in human oesophageal epithelium
- PMID: 9301492
- PMCID: PMC1891457
- DOI: 10.1136/gut.41.2.156
Biochemical analysis of the stress protein response in human oesophageal epithelium
Abstract
Background: The oesophageal epithelium is exposed routinely to noxious agents in the environment, including gastric acid, thermal stress, and chemical toxins. These epithelial cells have presumably evolved effective protective mechanisms to withstand tissue damage and repair injured cells. Heat shock protein or stress protein responses play a central role in protecting distinct cell types from different types of injury.
Aim: To determine (i) whether biochemical analysis of stress protein responses in pinch biopsy specimens from human oesophageal epithelium is feasible; (ii) whether undue stresses are imposed on cells by the act of sample collection, thus precluding analysis of stress responses; and (iii) if amenable to experimentation, the type of heat shock protein (Hsp) response that operates in the human oesophageal epithelium.
Methods: Tissue from the human oesophagus comprised predominantly of squamous epithelium was acquired within two hours of biopsy and subjected to an in vitro heat shock. Soluble tissue cell lysates derived from untreated or heat shocked samples were examined using denaturing polyacrylamide gel electrophoresis for changes in: (i) the pattern of general protein synthesis by labelling epithelial cells with 35S-methionine and (ii) the levels of soluble Hsp70 protein and related isoforms using immunochemical protein blots.
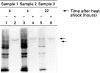
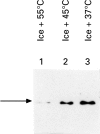
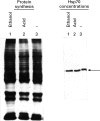
Results: A single pinch biopsy specimen is sufficient to extract and analyse specific sets of polypeptides in the oesophageal epithelium. After ex vivo heat shock, a classic inhibition of general protein synthesis is observed and correlates with the increased synthesis of two major proteins of molecular weight of 60 and 70 kDa. Notably, cells from unheated controls exhibit a "stressed" biochemical state 22 hours after incubation at 37 degrees C, as shown by inhibition of general protein synthesis and increased synthesis of the 70 kDa protein. These data indicate that only freshly acquired specimens are suitable for studying stress responses ex vivo. No evidence was found that the two heat induced polypeptides are previously identified Hsp70 isoforms. In fact, heat shock results in a reduction in the steady state concentrations of Hsp70 protein in the oesophageal epithelium.
Conclusion: Systematic and highly controlled studies on protein biochemistry are possible on epithelial biopsy specimens from the human oesophagus. These technical innovations have permitted the discovery of a novel heat shock response operating in the oesophageal epithelium. Notably, two polypeptides were synthesised after heat shock that seem to differ from Hsp70 protein. In addition, the striking reduction in steady state concentrations of Hsp70 protein after heat shock suggests that oesophageal epithelium has evolved an atypical biochemical response to thermal stress.
Figures





Comment in
-
Stress protein response in human oesophageal epithelium may be influenced by the ex vivo culture technique.Gut. 1998 Feb;42(2):307-8. doi: 10.1136/gut.42.2.307. Gut. 1998. PMID: 9536961 Free PMC article. No abstract available.
Similar articles
-
Decreased levels of heat shock proteins in gut epithelial cells after exposure to plant lectins.Gut. 2000 May;46(5):679-87. doi: 10.1136/gut.46.5.680. Gut. 2000. PMID: 10764712 Free PMC article.
-
The human oesophageal squamous epithelium exhibits a novel type of heat shock protein response.Eur J Biochem. 2001 Oct;268(20):5343-55. doi: 10.1046/j.0014-2956.2001.02468.x. Eur J Biochem. 2001. PMID: 11606197
-
Hsp56: a novel heat shock protein associated with untransformed steroid receptor complexes.J Biol Chem. 1990 Dec 25;265(36):22067-70. J Biol Chem. 1990. PMID: 2266108
-
The heat shock stress response after brain lesions: induction of 72 kDa heat shock protein (cell types involved, axonal transport, transcriptional regulation) and protein synthesis inhibition.Prog Neurobiol. 1997 Apr;51(6):607-36. doi: 10.1016/s0301-0082(97)00004-x. Prog Neurobiol. 1997. PMID: 9175159 Review.
-
The mammalian heat shock (or stress) response: a cellular defense mechanism.Adv Exp Med Biol. 1987;225:287-304. doi: 10.1007/978-1-4684-5442-0_26. Adv Exp Med Biol. 1987. PMID: 3331062 Review.
Cited by
-
Novel phosphorylation sites of human tumour suppressor protein p53 at Ser20 and Thr18 that disrupt the binding of mdm2 (mouse double minute 2) protein are modified in human cancers.Biochem J. 1999 Aug 15;342 ( Pt 1)(Pt 1):133-41. Biochem J. 1999. PMID: 10432310 Free PMC article.
-
Decreased levels of heat shock proteins in gut epithelial cells after exposure to plant lectins.Gut. 2000 May;46(5):679-87. doi: 10.1136/gut.46.5.680. Gut. 2000. PMID: 10764712 Free PMC article.
-
Desmin aggregate formation by R120G alphaB-crystallin is caused by altered filament interactions and is dependent upon network status in cells.Mol Biol Cell. 2004 May;15(5):2335-46. doi: 10.1091/mbc.e03-12-0893. Epub 2004 Mar 5. Mol Biol Cell. 2004. PMID: 15004226 Free PMC article.
-
Adaptive evolution of a stress response protein.PLoS One. 2007 Oct 10;2(10):e1003. doi: 10.1371/journal.pone.0001003. PLoS One. 2007. PMID: 17925851 Free PMC article.
-
An animal model to evaluate the function and regulation of the adaptively evolving stress protein SEP53 in oesophageal bile damage responses.Cell Stress Chaperones. 2008 Sep;13(3):375-85. doi: 10.1007/s12192-008-0037-1. Epub 2008 May 9. Cell Stress Chaperones. 2008. PMID: 18465210 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
