Evidence for the presence of multilineage chimerism and progenitors of donor dendritic cells in the peripheral blood of bone marrow-augmented organ transplant recipients
- PMID: 9311712
- PMCID: PMC2963997
- DOI: 10.1097/00007890-199709150-00013
Evidence for the presence of multilineage chimerism and progenitors of donor dendritic cells in the peripheral blood of bone marrow-augmented organ transplant recipients
Abstract
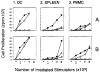
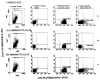
We have postulated that the donor leukocyte microchimerism plays a seminal role in the acceptance of allografts by inducing and perpetuating variable degree of donor-specific nonreactivity in long-surviving organ recipients. Limited information is available, however, concerning the phenotype and function of these chimeric cells in humans. The unequivocal presence of donor dendritic cells (DCs), a prominent lineage in the microchimerism observed in rodents and clinical organ recipients, was difficult to demonstrate in bone marrow (BM)-augmented organ transplant recipients. This enigma was resolved by the recent description of a method for propagating circulating human DCs from their progenitors by culture in a medium enriched with granulocyte-macrophage colony-stimulating factor and interleukin 4, a condition known to inhibit outgrowth of monocytes, thus providing a selective growth advantage to committed progenitors of the myeloid lineage. Cells from BM-augmented organ recipients and normal control subjects harvested from 12- to 14-day cultures exhibited dendritic morphology and potent allostimulatory capacity. Using appropriate primers, the presence of donor DNA was verified by polymerase chain reaction within the lineage(null)/class II(bright) sorted DC. Phenotypic analysis of cultured DCs from BM-augmented patients, unlike that of controls, exhibited a marked down-regulation of B7-1 (CD80) while retaining normal levels of expression of B7-2 (CD86) cell surface molecules. The presence of donor DNA was also confirmed by polymerase chain reaction in individually sorted lineage+ (T, B, and NK) cells and macrophages, suggesting that the chimerism in BM-augmented patients is multilineage. The presence of progenitors of donor DCs in the peripheral blood of BM-augmented patients further substantiates the already convincing evidence of stem cell engraftment.
Figures




Similar articles
-
Increased apoptosis of immunoreactive host cells and augmented donor leukocyte chimerism, not sustained inhibition of B7 molecule expression are associated with prolonged cardiac allograft survival in mice preconditioned with immature donor dendritic cells plus anti-CD40L mAb.Transplantation. 1999 Sep 27;68(6):747-57. doi: 10.1097/00007890-199909270-00006. Transplantation. 1999. PMID: 10515374 Free PMC article.
-
Microchimerism, dendritic cell progenitors and transplantation tolerance.Stem Cells. 1995 Nov;13(6):622-39. doi: 10.1002/stem.5530130607. Stem Cells. 1995. PMID: 8590864 Free PMC article. Review.
-
Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen.J Exp Med. 1994 Jun 1;179(6):1823-34. doi: 10.1084/jem.179.6.1823. J Exp Med. 1994. PMID: 8195710 Free PMC article.
-
Blockade of the CD40-CD40 ligand pathway potentiates the capacity of donor-derived dendritic cell progenitors to induce long-term cardiac allograft survival.Transplantation. 1997 Dec 27;64(12):1808-15. doi: 10.1097/00007890-199712270-00031. Transplantation. 1997. PMID: 9422424
-
Cellular basis of long-term organ transplant acceptance: pivotal role of intrathymic clonal deletion and thymic dependence of bone marrow microchimerism-associated tolerance.Am J Kidney Dis. 1998 Feb;31(2):197-212. doi: 10.1053/ajkd.1998.v31.pm9469488. Am J Kidney Dis. 1998. PMID: 9469488 Review.
Cited by
-
Translating transplantation tolerance in the clinic: where are we, where do we go?Clin Exp Immunol. 2009 May;156(2):185-8. doi: 10.1111/j.1365-2249.2009.03887.x. Epub 2009 Jan 22. Clin Exp Immunol. 2009. PMID: 19250278 Free PMC article. Review.
-
Dendritic cells and immune regulation in the liver.Gut. 2003 Feb;52(2):307-14. doi: 10.1136/gut.52.2.307. Gut. 2003. PMID: 12524419 Free PMC article. Review.
-
Human dendritic cells and transplant outcome.Transplantation. 2008 Jun 15;85(11):1513-22. doi: 10.1097/TP.0b013e318173a768. Transplantation. 2008. PMID: 18551047 Free PMC article. Review.
-
TIGIT+ iTregs elicited by human regulatory macrophages control T cell immunity.Nat Commun. 2018 Jul 20;9(1):2858. doi: 10.1038/s41467-018-05167-8. Nat Commun. 2018. PMID: 30030423 Free PMC article.
-
Microchimerism in promoting graft acceptance in clinical transplantation.Curr Opin Organ Transplant. 2011 Aug;16(4):345-52. doi: 10.1097/MOT.0b013e3283489a42. Curr Opin Organ Transplant. 2011. PMID: 21666474 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

