Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events
- PMID: 9314529
- PMCID: PMC2139822
- DOI: 10.1083/jcb.139.1.63
Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events
Abstract
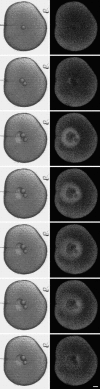
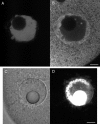
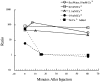
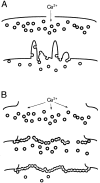
A microneedle puncture of the fibroblast or sea urchin egg surface rapidly evokes a localized exocytotic reaction that may be required for the rapid resealing that follows this breach in plasma membrane integrity (Steinhardt, R.A,. G. Bi, and J.M. Alderton. 1994. Science (Wash. DC). 263:390-393). How this exocytotic reaction facilitates the resealing process is unknown. We found that starfish oocytes and sea urchin eggs rapidly reseal much larger disruptions than those produced with a microneedle. When an approximately 40 by 10 microm surface patch was torn off, entry of fluorescein stachyose (FS; 1, 000 mol wt) or fluorescein dextran (FDx; 10,000 mol wt) from extracellular sea water (SW) was not detected by confocal microscopy. Moreover, only a brief (approximately 5-10 s) rise in cytosolic Ca2+ was detected at the wound site. Several lines of evidence indicate that intracellular membranes are the primary source of the membrane recruited for this massive resealing event. When we injected FS-containing SW deep into the cells, a vesicle formed immediately, entrapping within its confines most of the FS. DiI staining and EM confirmed that the barrier delimiting injected SW was a membrane bilayer. The threshold for vesicle formation was approximately 3 mM Ca2+ (SW is approximately 10 mM Ca2+). The capacity of intracellular membranes for sealing off SW was further demonstrated by extruding egg cytoplasm from a micropipet into SW. A boundary immediately formed around such cytoplasm, entrapping FDx or FS dissolved in it. This entrapment did not occur in Ca2+ -free SW (CFSW). When egg cytoplasm stratified by centrifugation was exposed to SW, only the yolk platelet-rich domain formed a membrane, suggesting that the yolk platelet is a critical element in this response and that the ER is not required. We propose that plasma membrane disruption evokes Ca2+ regulated vesicle-vesicle (including endocytic compartments but possibly excluding ER) fusion reactions. The function in resealing of this cytoplasmic fusion reaction is to form a replacement bilayer patch. This patch is added to the discontinuous surface bilayer by exocytotic fusion events.
Figures







References
-
- Blinc A, Francis CE. Transport processes in fibrinolysis and fibrinolytic therapy. Thromb Haemostasis. 1996;76:481–491. - PubMed
-
- Casademont J, Carpenter S, Karpati G. Vacuolation of muscle fibers near sarcolemmal breaks represents T tubule dilatation secondary to enhanced sodium pump activity. J Neuropathol Exp Neurol. 1988;47:618–628. - PubMed
-
- Chambers, R., and E.L. Chambers. 1961. Explorations into the Nature of the Living Cell. Harvard University Press, Cambridge, MA. 352 pp.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

