Induction of the angiogenic phenotype by Hox D3
- PMID: 9314544
- PMCID: PMC2139816
- DOI: 10.1083/jcb.139.1.257
Induction of the angiogenic phenotype by Hox D3
Abstract
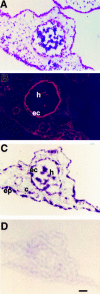
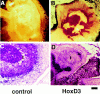
Angiogenesis is characterized by distinct phenotypic changes in vascular endothelial cells (EC). Evidence is provided that the Hox D3 homeobox gene mediates conversion of endothelium from the resting to the angiogenic/invasive state. Stimulation of EC with basic fibroblast growth factor (bFGF) resulted in increased expression of Hox D3, integrin alphavbeta3, and the urokinase plasminogen activator (uPA). Hox D3 antisense blocked the ability of bFGF to induce uPA and integrin alphavbeta3 expression, yet had no effect on EC cell proliferation or bFGF-mediated cyclin D1 expression. Expression of Hox D3, in the absence of bFGF, resulted in enhanced expression of integrin alphavbeta3 and uPA. In fact, sustained expression of Hox D3 in vivo on the chick chorioallantoic membrane retained EC in this invasive state and prevented vessel maturation leading to vascular malformations and endotheliomas. Therefore, Hox D3 regulates EC gene expression associated with the invasive stage of angiogenesis.
Figures








References
-
- Bautch VL, Toda S, Hassell JA, Hanahan D. Endothelial cell tumors develop in transgenic mice carrying polyoma middle T oncogene. Cell. 1987;51:529–538. - PubMed
-
- Botas J. Control of morphogenesis and differentiation by HOM/Hoxgenes. Curr Opin Cell Biol. 1993;5:1015–1022. - PubMed
-
- Brooks PC, Clark RAF, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science (Wash DC) 1994;264:569–571. - PubMed
-
- Brooks PC, Strömblad S, Sanders L, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. - PubMed
-
- Brown WM, Zhou L, Taylor GR. The nucleotide sequence of the murine Hox D3gene reveals extensive identity with the human protein. Biochim Biophys Acta. 1994;1219:219–222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

