Beta 1 integrin is essential for teratoma growth and angiogenesis
- PMID: 9314545
- PMCID: PMC2139829
- DOI: 10.1083/jcb.139.1.265
Beta 1 integrin is essential for teratoma growth and angiogenesis
Abstract
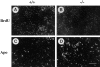
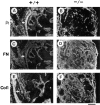
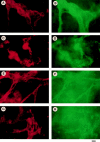
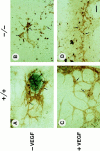
Teratomas are benign tumors that form after ectopic injection of embryonic stem (ES) cells into mice and contain derivatives of all primitive germ layers. To study the role of beta 1 integrin during teratoma formation, we compared teratomas induced by normal and beta1-null ES cells. Injection of normal ES cells gave rise to large teratomas. In contrast, beta 1-null ES cells either did not grow or formed small teratomas with an average weight of <5% of that of normal teratomas. Histological analysis of beta 1-null teratomas revealed the presence of various differentiated cells, however, a much lower number of host-derived stromal cells than in normal teratomas. Fibronectin, collagen I, and nidogen were expressed but, in contrast to normal teratomas, diffusely deposited in beta1-null teratomas. Basement membranes were present but with irregular shape and detached from the cell surface. Normal teratomas had large blood vessels with a smooth inner surface, containing both host- and ES cell-derived endothelial cells. In contrast, beta 1-null teratomas had small vessels that were loosely embedded into the connective tissue. Furthermore, endothelial cells were always of host-derived origin and formed blood vessels with an irregular inner surface. Although beta 1- deficient endothelial cells were absent in teratomas, beta 1-null ES cells could differentiate in vitro into endothelial cells. The formation of a complex vasculature, however, was significantly delayed and of poor quality in beta1-null embryoid bodies. Moreover, while vascular endothelial growth factor induced proliferation of endothelial cells as well as an extensive branching of blood vessels in normal embryoid bodies, it had no effect in beta 1-null embryoid bodies.
Figures










References
-
- Alexander, C.M., and Z. Werb. 1991. Degradation of extracellular matrix. In Cell Biology of Extracellular Matrix. E. Hay, editor. Plenum Press, New York. pp. 255–294.
-
- Bagutti C, Wobus AM, Fässler R, Watt FM. Differentiation of embryonal stem cells into keratinocytes: comparison of wild-type and β1 integrin-deficient cells. Dev Biol. 1996;179:184–196. - PubMed
-
- Brooks PC, Clark RAF, Cheresch DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science (Wash DC) 1994a;264:569–571. - PubMed
-
- Brooks PC, Montgomery AMP, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin αvβ3antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994b;79:1157–1164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

