Gastric hyperplasia and increased proliferative responses of lymphocytes in mice lacking the COOH-terminal ankyrin domain of NF-kappaB2
- PMID: 9314550
- PMCID: PMC2199059
- DOI: 10.1084/jem.186.7.999
Gastric hyperplasia and increased proliferative responses of lymphocytes in mice lacking the COOH-terminal ankyrin domain of NF-kappaB2
Abstract
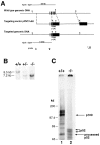
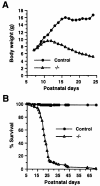
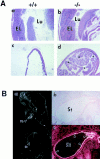
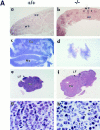
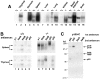
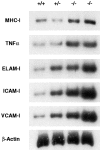
The nfkb2 gene encodes the p100 precursor which produces the p52 protein after proteolytic cleavage of its COOH-terminal domain. Although the p52 product can act as an alternative subunit of NF-kappaB, the p100 precursor is believed to function as an inhibitor of Rel/NF-kappaB activity by cytoplasmic retention of Rel/NF-kappaB complexes, like other members of the IkappaB family. However, the physiological relevance of the p100 precursor as an IkappaB molecule has not been understood. To assess the role of the precursor in vivo, we generated, by gene targeting, mice lacking p100 but still containing a functional p52 protein. Mice with a homozygous deletion of the COOH-terminal ankyrin repeats of NF-kappaB2 (p100(-/-)) had marked gastric hyperplasia, resulting in early postnatal death. p100(-/-) animals also presented histopathological alterations of hematopoietic tissues, enlarged lymph nodes, increased lymphocyte proliferation in response to several stimuli, and enhanced cytokine production in activated T cells. Dramatic induction of nuclear kappaB-binding activity composed of p52-containing complexes was found in all tissues examined and also in stimulated lymphocytes. Thus, the p100 precursor is essential for the proper regulation of p52-containing Rel/NF-kappaB complexes in various cell types and its absence cannot be efficiently compensated for by other IkappaB proteins.
Figures
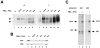
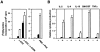
Similar articles
-
p100, a precursor of NF-κB2, inhibits c-Rel and reduces the expression of IL-23 in dendritic cells.Biochem Biophys Res Commun. 2014 Oct 24;453(3):332-7. doi: 10.1016/j.bbrc.2014.09.143. Epub 2014 Oct 8. Biochem Biophys Res Commun. 2014. PMID: 25305492
-
Multiple hemopoietic defects and lymphoid hyperplasia in mice lacking the transcriptional activation domain of the c-Rel protein.J Exp Med. 1998 Apr 6;187(7):973-84. doi: 10.1084/jem.187.7.973. J Exp Med. 1998. PMID: 9529314 Free PMC article.
-
CD40 regulates the processing of NF-kappaB2 p100 to p52.EMBO J. 2002 Oct 15;21(20):5375-85. doi: 10.1093/emboj/cdf542. EMBO J. 2002. PMID: 12374738 Free PMC article.
-
A key role for NF-κB transcription factor c-Rel in T-lymphocyte-differentiation and effector functions.Clin Dev Immunol. 2012;2012:239368. doi: 10.1155/2012/239368. Epub 2012 Mar 6. Clin Dev Immunol. 2012. PMID: 22481964 Free PMC article. Review.
-
The involvement of the candidate proto-oncogene NFKB2/lyt-10 in lymphoid malignancies.Leuk Lymphoma. 1996 Sep;23(1-2):43-8. doi: 10.3109/10428199609054800. Leuk Lymphoma. 1996. PMID: 9021684 Review.
Cited by
-
Stat3 activation of NF-{kappa}B p100 processing involves CBP/p300-mediated acetylation.Proc Natl Acad Sci U S A. 2006 May 9;103(19):7264-9. doi: 10.1073/pnas.0509808103. Epub 2006 May 1. Proc Natl Acad Sci U S A. 2006. PMID: 16651533 Free PMC article.
-
Substrate complex competition is a regulatory motif that allows NFκB RelA to license but not amplify NFκB RelB.Proc Natl Acad Sci U S A. 2019 May 21;116(21):10592-10597. doi: 10.1073/pnas.1816000116. Epub 2019 May 2. Proc Natl Acad Sci U S A. 2019. PMID: 31048505 Free PMC article.
-
Regulation of immune responses by NF-kappa B/Rel transcription factor.J Exp Med. 1998 Jan 19;187(2):143-6. doi: 10.1084/jem.187.2.143. J Exp Med. 1998. PMID: 9432972 Free PMC article. Review. No abstract available.
-
Novel nonsense gain-of-function NFKB2 mutations associated with a combined immunodeficiency phenotype.Blood. 2017 Sep 28;130(13):1553-1564. doi: 10.1182/blood-2017-05-782177. Epub 2017 Aug 4. Blood. 2017. PMID: 28778864 Free PMC article.
-
Lymphotoxin-β receptor signaling through NF-κB2-RelB pathway reprograms adipocyte precursors as lymph node stromal cells.Immunity. 2012 Oct 19;37(4):721-34. doi: 10.1016/j.immuni.2012.06.010. Epub 2012 Aug 30. Immunity. 2012. PMID: 22940098 Free PMC article.
References
-
- Beg AA, Baldwin AS., Jr The IκB proteins: multifunctional regulators of Rel/NF-κB transcription factors. Genes Dev. 1993;7:2063–2070. - PubMed
-
- Gilmore TD, Morin PJ. The IκB proteins: members of a multifunctional family. Trends Genet. 1993;9:427–433. - PubMed
-
- Liou HC, Baltimore D. Regulation of the NF-κB/rel transcription factor and IκB inhibitor system. Curr Opin Cell Biol. 1993;5:477–487. - PubMed
-
- Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-κB. Annu Rev Cell Biol. 1994;10:405–455. - PubMed
-
- Finco TS, Baldwin AS. Mechanistic aspects of NF-κB regulation: the emerging role of phosphorylation and proteolysis. Immunity. 1995;3:263–272. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

