A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages
- PMID: 9314552
- PMCID: PMC2199061
- DOI: 10.1084/jem.186.7.1027
A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages
Abstract
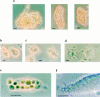
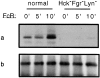
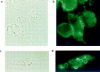
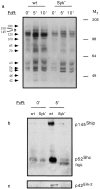
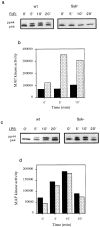
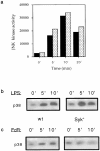
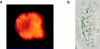
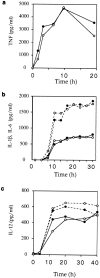
Receptors on macrophages for the Fc region of IgG (FcgammaR) mediate a number of responses important for host immunity. Signaling events necessary for these responses are likely initiated by the activation of Src-family and Syk-family tyrosine kinases after FcgammaR cross-linking. Macrophages derived from Syk-deficient (Syk-) mice were defective in phagocytosis of particles bound by FcgammaRs, as well as in many FcgammaR-induced signaling events, including tyrosine phosphorylation of a number of cellular substrates and activation of MAP kinases. In contrast, Syk- macrophages exhibited normal responses to another potent macrophage stimulus, lipopolysaccharide. Phagocytosis of latex beads and Escherichia coli bacteria was also not affected. Syk- macrophages exhibited formation of polymerized actin structures opposing particles bound to the cells by FcgammaRs (actin cups), but failed to proceed to internalization. Interestingly, inhibitors of phosphatidylinositol 3-kinase also blocked FcgammaR-mediated phagocytosis at this stage. Thus, PI 3-kinase may participate in a Syk-dependent signaling pathway critical for FcgammaR-mediated phagocytosis. Macrophages derived from mice deficient for the three members of the Src-family of kinases expressed in these cells, Hck, Fgr, and Lyn, exhibited poor Syk activation upon FcgammaR engagement, accompanied by a delay in FcgammaR-mediated phagocytosis. These observations demonstrate that Syk is critical for FcgammaR-mediated phagocytosis, as well as for signal transduction in macrophages. Additionally, our findings provide evidence to support a model of sequential tyrosine kinase activation by FcgammaR's analogous to models of signaling by the B and T cell antigen receptors.
Figures



References
-
- Ravetch JV, Kinet JP. Fc receptors. Ann Rev Immunol. 1991;9:457–492. - PubMed
-
- Brooks D, Ravetch JV. Fc receptor signaling. Adv Exp Med Biol. 1994;365:185–195. - PubMed
-
- Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. - PubMed
-
- Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature (Lond) 1996;379:346–349. - PubMed
-
- Ravetch JV. Fc receptors: rubor redux. Cell. 1994;78:553–560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

