Two novel routes of transporter associated with antigen processing (TAP)-independent major histocompatibility complex class I antigen processing
- PMID: 9314557
- PMCID: PMC2199067
- DOI: 10.1084/jem.186.7.1087
Two novel routes of transporter associated with antigen processing (TAP)-independent major histocompatibility complex class I antigen processing
Abstract
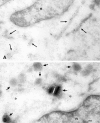
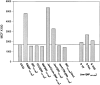
Jaw1 is an endoplasmic reticulum (ER) resident protein representative of a class of proteins post translationally inserted into membranes via a type II membrane anchor (cytosolic NH2 domain, lumenal COOH domain) in a translocon-independent manner. We found that Jaw1 can efficiently deliver a COOH-terminal antigenic peptide to class I molecules in transporter associated with antigen processing (TAP)-deficient cells or cells in which TAP is inactivated by the ICP47 protein. Peptide delivery mediated by Jaw1 to class I molecules was equal or better than that mediated by the adenovirus E3/19K glycoprotein signal sequence, and was sufficient to enable cytofluorographic detection of newly recruited thermostabile class I molecules at the surface of TAP-deficient cells. Deletion of the transmembrane region retargeted Jaw1 from the ER to the cytosol, and severely, although incompletely, abrogated its TAP-independent peptide carrier activity. Use of different protease inhibitors revealed the involvement of a nonproteasomal protease in the TAP-independent activity of cytosolic Jaw1. These findings demonstrate two novel TAP-independent routes of antigen processing; one based on highly efficient peptide liberation from the COOH terminus of membrane proteins in the ER, the other on delivery of a cytosolic protein to the ER by an unknown route.
Figures
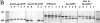
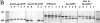




Similar articles
-
A viral, transporter associated with antigen processing (TAP)-independent, high affinity ligand with alternative interactions endogenously presented by the nonclassical human leukocyte antigen E class I molecule.J Biol Chem. 2012 Oct 12;287(42):34895-34903. doi: 10.1074/jbc.M112.362293. Epub 2012 Aug 27. J Biol Chem. 2012. PMID: 22927436 Free PMC article.
-
TAP (transporter associated with antigen processing)-independent presentation of endogenously synthesized peptides is enhanced by endoplasmic reticulum insertion sequences located at the amino- but not carboxyl-terminus of the peptide.J Immunol. 1994 Jan 15;152(2):381-7. J Immunol. 1994. PMID: 8283027
-
Exogenous peptides enter the endoplasmic reticulum of TAP-deficient cells and induce the maturation of nascent MHC class I molecules.Eur J Immunol. 2001 Apr;31(4):1181-90. doi: 10.1002/1521-4141(200104)31:4<1181::aid-immu1181>3.0.co;2-j. Eur J Immunol. 2001. PMID: 11298343
-
TAP-independent delivery of antigenic peptides to the endoplasmic reticulum: therapeutic potential and insights into TAP-dependent antigen processing.J Immunother. 1998 Mar;21(2):127-31. doi: 10.1097/00002371-199803000-00006. J Immunother. 1998. PMID: 9551364 Review.
-
Endogenous TAP-independent MHC-I antigen presentation: not just the ER lumen.Curr Opin Immunol. 2020 Jun;64:9-14. doi: 10.1016/j.coi.2019.12.003. Epub 2020 Jan 11. Curr Opin Immunol. 2020. PMID: 31935516 Review.
Cited by
-
The same well-characterized T cell epitope SIINFEKL expressed in the context of a cytoplasmic or secreted protein in BCG induces different CD8+ T cell responses.Immunol Lett. 2010 May 4;130(1-2):36-42. doi: 10.1016/j.imlet.2009.12.004. Epub 2009 Dec 11. Immunol Lett. 2010. PMID: 20005257 Free PMC article.
-
Generation of MHC class I ligands in the secretory and vesicular pathways.Cell Mol Life Sci. 2011 May;68(9):1543-52. doi: 10.1007/s00018-011-0661-2. Epub 2011 Mar 9. Cell Mol Life Sci. 2011. PMID: 21387141 Free PMC article. Review.
-
Varied Role of Ubiquitylation in Generating MHC Class I Peptide Ligands.J Immunol. 2017 May 15;198(10):3835-3845. doi: 10.4049/jimmunol.1602122. Epub 2017 Mar 31. J Immunol. 2017. PMID: 28363906 Free PMC article.
-
Alternative Antigen Processing for MHC Class I: Multiple Roads Lead to Rome.Front Immunol. 2015 Jun 5;6:298. doi: 10.3389/fimmu.2015.00298. eCollection 2015. Front Immunol. 2015. PMID: 26097483 Free PMC article. Review.
-
Detection of Quiescent Radioresistant Epithelial Progenitors in the Adult Thymus.Front Immunol. 2017 Dec 5;8:1717. doi: 10.3389/fimmu.2017.01717. eCollection 2017. Front Immunol. 2017. PMID: 29259606 Free PMC article.
References
-
- Townsend A, Bodmer H. Antigen recognition by class I–restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. - PubMed
-
- Yewdell JW, Bennink JR. Cell biology of antigen processing and presentation to MHC class I molecule-restricted T lymphocytes. Adv Immunol. 1992;52:1–123. - PubMed
-
- Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. - PubMed
-
- Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. - PubMed
-
- Goldberg AL, Rock KL. Proteolysis, proteasomes and antigen processing. Nature (Lond) 1992;357:375–379. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

