Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors
- PMID: 9314567
- PMCID: PMC2199074
- DOI: 10.1084/jem.186.7.1177
Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors
Abstract
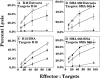
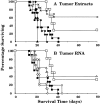
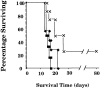
Recent studies have shown that the brain is not a barrier to successful active immunotherapy that uses gene-modified autologous tumor cell vaccines. In this study, we compared the efficacy of two types of vaccines for the treatment of tumors within the central nervous system (CNS): dendritic cell (DC)-based vaccines pulsed with either tumor extract or tumor RNA, and cytokine gene-modified tumor vaccines. Using the B16/F10 murine melanoma (B16) as a model for CNS tumor, we show that vaccination with bone marrow-generated DCs, pulsed with either B16 cell extract or B16 total RNA, can induce specific cytotoxic T lymphocytes against B16 tumor cells. Both types of DC vaccines were able to protect animals from tumors located in the CNS. DC-based vaccines also led to prolonged survival in mice with tumors placed before the initiation of vaccine therapy. The DC-based vaccines were at least as effective, if not more so, as vaccines containing B16 tumor cells in which the granulocytic macrophage colony-stimulating factor gene had been modified. These data support the use of DC-based vaccines for the treatment of patients with CNS tumors.
Figures


References
-
- Grooms GA, Eilber FR, Morton DL. Failure of adjuvant immunotherapy to prevent central nervous system metastases in malignant melanoma patients. J Surg Oncol. 1977;9:147–153. - PubMed
-
- Mitchell MS. Relapse in the central nervous system in melanoma patients successfully treated with biomodulators. J Clin Oncol. 1989;7:1701–1709. - PubMed
-
- Sampson JH, Archer GE, Ashley DM, Fuchs HE, Hale LP, Dranoff G, Bigner DD. Subcutaneous vaccination with irradiated, cytokine-producing tumor cells stimulates CD8+cell mediated immunity against tumors located in the “immunologically privileged” central nervous system. Proc Natl Acad Sci USA. 1996;93:10399–10404. - PMC - PubMed
-
- Bigner DD, Pitts OM, Wikstrand CJ. Induction of lethal experimental allergic encephalomyelitis in nonhuman primates and guinea pigs with human glioblastoma multiforme tissue. J Neurosurg. 1981;55:32–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

