Neurons promote the translocation of peripheral myelin protein 22 into myelin
- PMID: 9315897
- PMCID: PMC6793898
- DOI: 10.1523/JNEUROSCI.17-20-07754.1997
Neurons promote the translocation of peripheral myelin protein 22 into myelin
Abstract
Schwann cells express low levels of myelin proteins in the absence of neurons. When Schwann cells and neurons are cultured together the production of myelin proteins is elevated, and myelin is formed. For peripheral myelin protein 22 (PMP22), the exact amount of protein produced is critical, because peripheral neuropathies result from its underexpression or overexpression. In this study we examined the effect of neurons on Schwann cell PMP22 production in culture and in peripheral nerve using metabolic labeling and pulse-chase studies as well as immunocytochemistry. Most of the newly synthesized PMP22 in Schwann cells is rapidly degraded in the endoplasmic reticulum. Only a small proportion of the total PMP22 acquires complex glycosylation and accumulates in the Golgi compartment. This material is translocated to the Schwann cell membrane in detectable amounts only when axonal contact and myelination occur. Myelination does not, however, alter the rapid turnover of PMP22 in Schwann cells. PMP22 may therefore be a unique myelin protein in that axonal contact promotes its insertion into the Schwann cell membrane and myelin without altering its rapid turnover rate within the cell.
Figures
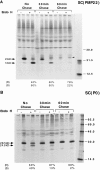


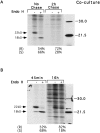
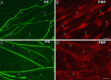
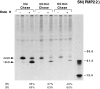
References
-
- Adlkofer K, Martini R, Aguzzi A, Zielasek J, Toyka KV, Suter U. Hypermyelination and demyelinating peripheral neuropathy in Pmp22-deficient mice. Nat Genet. 1995;11:274–280. - PubMed
-
- Bosse F, Zoidl G, Wilms S, Gillen CP, Kuhn HG, Müller HW. Differential expression of two mRNA species indicates a dual function of peripheral myelin protein PMP22 in cell growth and myelination. J Neurosci Res. 1994;37:529–537. - PubMed
-
- Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. - PubMed
-
- Brunden KR. Age-dependent changes in the oligosaccharide structure of the major myelin glycoprotein, P0. J Neurochem. 1992;58:1659–1666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
