Pattern deformities and cell loss in Engrailed-2 mutant mice suggest two separate patterning events during cerebellar development
- PMID: 9315908
- PMCID: PMC6793924
- DOI: 10.1523/JNEUROSCI.17-20-07881.1997
Pattern deformities and cell loss in Engrailed-2 mutant mice suggest two separate patterning events during cerebellar development
Abstract
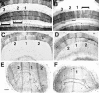
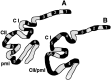
Null alleles of the mouse Engrailed-2 gene, a molecular homolog of the fly gene engrailed, have demonstrable effects on the anteroposterior (A/P) patterning of cerebellum as reflected in the disruption of the normal process of foliation of the cerebellar cortex and the alteration of transgene expression boundaries in the adult. Engrailed-2 also affects the transient mediolateral (M/L) pattern of En-1 and Wnt-7b expression seen in late embryogenesis. We have examined three markers of cerebellar compartmentation in En-2 mutant mice: the Zebrin II and Ppath monoclonal antibodies and the transgene L7lacZ. In En-2 mutants, the normal temporal pattern of expression is preserved for all three markers, although the size and spatial location of various bands differ from those of the wild type. Unlike the foliation abnormalities, the M/L pattern disturbances we have found occur in nearly all cerebellar regions. Cell counts reveal that all major cell types of the olivocerebellar circuit are reduced by 30-40%. We propose that these results are best explained by a model in which the Engrailed-2 gene is involved in the early specification of the cerebellar field including the number of progenitors. Because each of these progenitors gives rise to a clone of defined size, Engrailed-2 helps specify adult cell number. We further postulate that the configuration of the seven Zebrin bands as well as the shapes and locations of the cerebellar lobules are set up by a second patterning event that occurs after neurogenesis is complete.
Figures




Similar articles
-
Ectopic overexpression of engrailed-2 in cerebellar Purkinje cells causes restricted cell loss and retarded external germinal layer development at lobule junctions.J Neurosci. 1998 Mar 1;18(5):1763-73. doi: 10.1523/JNEUROSCI.18-05-01763.1998. J Neurosci. 1998. PMID: 9465001 Free PMC article.
-
Factors in the genetic background suppress the engrailed-1 cerebellar phenotype.J Neurosci. 2003 Jun 15;23(12):5105-12. doi: 10.1523/JNEUROSCI.23-12-05105.2003. J Neurosci. 2003. PMID: 12832534 Free PMC article.
-
Selective disruption of "late onset" sagittal banding patterns by ectopic expression of engrailed-2 in cerebellar Purkinje cells.J Neurosci. 1999 Jul 1;19(13):5370-9. doi: 10.1523/JNEUROSCI.19-13-05370.1999. J Neurosci. 1999. PMID: 10377347 Free PMC article.
-
Cell death as a regulator of cerebellar histogenesis and compartmentation.Cerebellum. 2011 Sep;10(3):373-92. doi: 10.1007/s12311-010-0222-5. Cerebellum. 2011. PMID: 20941559 Review.
-
Development of the olivocerebellar projection.Perspect Dev Neurobiol. 1997;5(1):57-67. Perspect Dev Neurobiol. 1997. PMID: 9509518 Review.
Cited by
-
Persistent engrailed expression is required to determine sensory axon trajectory, branching, and target choice.J Neurosci. 2002 Feb 1;22(3):832-41. doi: 10.1523/JNEUROSCI.22-03-00832.2002. J Neurosci. 2002. PMID: 11826113 Free PMC article.
-
Aberrant Somatosensory Processing and Connectivity in Mice Lacking Engrailed-2.J Neurosci. 2019 Feb 20;39(8):1525-1538. doi: 10.1523/JNEUROSCI.0612-18.2018. Epub 2018 Dec 28. J Neurosci. 2019. PMID: 30593497 Free PMC article.
-
Genetic control of the mouse cerebellum: identification of quantitative trait loci modulating size and architecture.J Neurosci. 2001 Jul 15;21(14):5099-109. doi: 10.1523/JNEUROSCI.21-14-05099.2001. J Neurosci. 2001. PMID: 11438585 Free PMC article.
-
Cerebellum morphogenesis: the foliation pattern is orchestrated by multi-cellular anchoring centers.Neural Dev. 2007 Dec 3;2:26. doi: 10.1186/1749-8104-2-26. Neural Dev. 2007. PMID: 18053187 Free PMC article.
-
Engrailed homeobox genes determine the organization of Purkinje cell sagittal stripe gene expression in the adult cerebellum.J Neurosci. 2008 Nov 19;28(47):12150-62. doi: 10.1523/JNEUROSCI.2059-08.2008. J Neurosci. 2008. PMID: 19020009 Free PMC article.
References
-
- Avarado-Mallart R-M, Martinez S, Lance-Jones C. Pluripotentiality of the 2 day old avian germinative neuroepithelium. Dev Biol. 1990;139:75–88. - PubMed
-
- Baader S, Schilling M, Rosengarten B, Pretsch W, Teutsch H, Oberdick J, Schilling K. Purkinje cell lineage and the topographic organization of the cerebellar cortex: a view from X inactivation mosaics. Dev Biol. 1996;174:393–406. - PubMed
-
- Blatt G, Eisenman L. A qualitative and quantitative light microscopic study of the inferior olivary complex of normal, reeler and weaver mutant mice. J Comp Neurol. 1985;232:117–128. - PubMed
-
- Caddy K, Biscoe T. Structural and quantitative studies on the normal C3H and Lurcher mutant mouse. Philos Trans R Soc Lond [Biol] 1979;287:167–201. - PubMed
-
- Davis C, Joyner A. Expression patterns of the homeobox-containing genes En-1 and En-2 and the protooncogene int-1 diverge during mouse development. Genes Dev. 1988;2:1736–1744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
