Organization of corticostriatal and corticoamygdalar projections arising from the anterior inferotemporal area TE of the macaque monkey: a Phaseolus vulgaris leucoagglutinin study
- PMID: 9315910
- PMCID: PMC6793888
- DOI: 10.1523/JNEUROSCI.17-20-07902.1997
Organization of corticostriatal and corticoamygdalar projections arising from the anterior inferotemporal area TE of the macaque monkey: a Phaseolus vulgaris leucoagglutinin study
Abstract
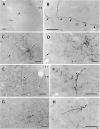
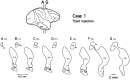
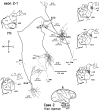
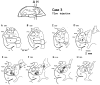
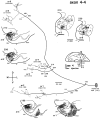
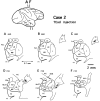
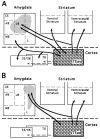
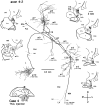
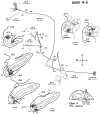
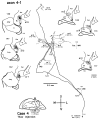
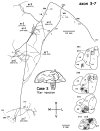
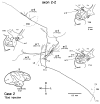
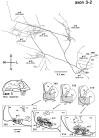
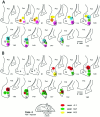
Corticostriatal and corticoamygdalar projections arising from area TE of the macaque monkey were studied by focal injections of the anterograde tracer Phaseolus vulgaris leucoagglutinin into the dorsolateral and ventromedial subdivisions of the anterior TE (TEad and TEav, respectively). This approach yielded several new results. First, the global distributions of labeled terminals revealed that both TEad and TEav projected to the ventrocaudal striatum, including the tail of the caudate nucleus and the adjacent ventral putamen, and the dorsolateral aspect of the deep amygdaloid nuclei. TEav also projected to the medial basal nucleus of the amygdala and the ventral striatum. Second, the reconstructed single axons (n = 18) demonstrated that some axons originating from TEav or TEad projected simultaneously to the ventrocaudal striatum and the dorsolateral aspect of the deep amygdaloid nuclei by giving off collaterals. TEav axons projected to the medial basal nucleus of the amygdala also had collaterals projecting to the perirhinal cortex or area TG. And third, it was revealed that the axons originating from a focal TEav or TEad projected to a restricted territory (3.4-3.6 mm rostrocaudally) in the ventrocaudal striatum with four to six dispersed, rostrocaudally elongated, rod-like modules. Individual axons with multiple arbors innervated many of these modules. These findings add the evidence that the anterior part of TE is anatomically heterogeneous and suggest that the deep amygdaloid nuclei may be functionally dissociated, with the dorsolateral aspect more closely related to the ventrocaudal striatum and the medial basal nucleus more closely related to the perirhinal cortex.
Figures





References
-
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta). Brain Res. 1980;190:347–368. - PubMed
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuit: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. - PubMed
-
- Amaral DG. Amygdalohippocampal and amygdalocortical projections in the primate brain. In: Schwarcz R, Ben-Ari Y, editors. Excitatory amino acids and epilepsy. Plenum; New York: 1986. pp. 3–17. - PubMed
-
- Amaral DG, Cowan WM. Subcortical afferents to the hippocampal formation in the monkey. J Comp Neurol. 1980;189:573–591. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
