Different mechanisms mediate development and expression of tolerance and dependence for peripheral mu-opioid antinociception in rat
- PMID: 9315920
- PMCID: PMC6793920
- DOI: 10.1523/JNEUROSCI.17-20-08018.1997
Different mechanisms mediate development and expression of tolerance and dependence for peripheral mu-opioid antinociception in rat
Abstract
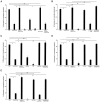
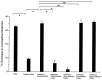
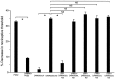
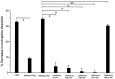
The mu-opioid [D-Ala2,N-Me-Phe4,Gly-ol5]-enkephalin (DAMGO) exerts a peripheral antinociceptive effect against prostaglandin E2 (PGE2)-induced mechanical hyperalgesia in the hindpaw of the rat. Tolerance and dependence develop to this effect. We have shown previously that tolerance and dependence can be dissociated and are mediated by different second messenger systems. In the present study, we evaluated whether the same or different second messenger systems mediate the development of this peripheral opioid tolerance or dependence compared with the expression of the loss of antinociceptive effect or rebound opioid antagonist hyperalgesia (i. e., expression of tolerance and dependence). DAMGO-induced tolerance was prevented by pretreatment with the nitric oxide synthase inhibitor NG-methyl-L-arginine (NMLA) but not by the protein kinase C (PKC) inhibitor chelerythrine, the adenylyl cyclase inhibitor 2',5'-dideoxyadenosine (ddA), or the calcium chelators 3,4,5-trimethoxybenzoic acid 8-(diethylamino)-octyl ester (TMB-8) and 2-[(2-bis-[carboxymethyl]amino-5-methylphenoxy)-methyl]-6-methoxy-8-bis [carboxymethyl]aminoquinoline (Quin-2). Once established, however, expression of DAMGO tolerance was acutely reversed by TMB-8 or Quin-2 but not by chelerythrine or NMLA. In contrast, naloxone-precipitated hyperalgesia in DAMGO-tolerant paws, a measure of dependence, was blocked by pretreatment with chelerythrine but not by NMLA, ddA, TMB-8, or Quin-2. Naloxone-precipitated hyperalgesia in DAMGO-tolerant paws was acutely reversed by chelerythrine, ddA, TMB-8, or Quin-2 but not by NMLA. Taken together, these results provide the first evidence that different mechanisms mediate the development and expression of both tolerance and dependence to the peripheral antinociceptive effect of DAMGO. However, although the development of tolerance and dependence are entirely separable, the expression of tolerance and dependence shares common calcium-dependent mechanisms.
Figures
References
-
- Adams ML, Kalicki JM, Meyer ER, Cicero TJ. Inhibition of the morphine withdrawal syndrome by a nitric oxide synthase inhibitor, NG-nitro-l-arginine methyl ester. Life Sci. 1993;52:PL245–PL249. - PubMed
-
- Alkon DL, Kubota M, Neary JT, Naito S, Coulter D, Ramussen H. C-kinase activation prolongs CA++-dependent inactivation of K+ currents. Biochem Biophys Res Commun. 1986;134:1245–1253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
