Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation
- PMID: 9315923
- PMCID: PMC6793895
- DOI: 10.1523/JNEUROSCI.17-20-08049.1997
Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation
Abstract
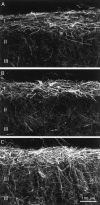
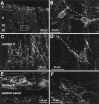
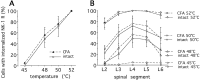
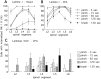
Although the neurokinin-1 (NK-1)/substance P (SP) receptor is expressed by neurons throughout the spinal dorsal horn, noxious chemical stimulation in the normal rat only induces internalization of the receptor in cell bodies and dendrites of lamina I. Here we compared the effects of mechanical and thermal stimulation in normal rats and in rats with persistent hindpaw inflammation. Electron microscopic analysis confirmed the upregulation of receptor that occurs with inflammation and demonstrated that in the absence of superimposed stimulation, the increased receptor was, as in normal rats, concentrated on the plasma membrane. In general, noxious mechanical was more effective than noxious thermal stimulation in inducing NK-1 receptor internalization, and this was increased in the setting of inflammation. Although a 5 sec noxious mechanical stimulus only induced internalization in 22% of lamina I neurons in normal rats, after inflammation, it evoked near-maximal (98%) internalization in lamina I, produced significant changes in laminae III-VI, and expanded the rostrocaudal distribution of neurons with internalized receptor. Even non-noxious (brush) stimulation of the inflamed hindpaw induced internalization in large numbers of superficial and deep neurons. For thermal stimulation, the percentage of cells with internalized receptor increased linearly at >45 degrees C, but in normal rats, these were restricted to lamina I. After inflammation, however, the 52 degrees C stimulus also induced internalization in 25% of laminae III-IV cells. These studies provide a new perspective on the reorganization of dorsal horn circuits in the setting of persistent injury and demonstrate a critical contribution of SP.
Figures




References
-
- Abbadie C, Brown JL, Mantyh PW, Basbaum AI. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–209. - PubMed
-
- Bleazard L, Hill RG, Morris R. The correlation between the distribution of the NK1 receptor and the actions of tachykinin agonists in the dorsal horn of the rat indicates that substance P does not have a functional role on substantia gelatinosa (lamina II) neurons. J Neurosci. 1994;14:7655–7664. - PMC - PubMed
-
- Boeynaems JM. Outlines of receptor theory. Elsevier; Amsterdam: 1980.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
