Decreased blood pressure response in mice deficient of the alpha1b-adrenergic receptor
- PMID: 9326654
- PMCID: PMC23548
- DOI: 10.1073/pnas.94.21.11589
Decreased blood pressure response in mice deficient of the alpha1b-adrenergic receptor
Abstract
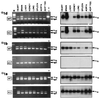
To investigate the functional role of different alpha1-adrenergic receptor (alpha1-AR) subtypes in vivo, we have applied a gene targeting approach to create a mouse model lacking the alpha1b-AR (alpha1b-/-). Reverse transcription-PCR and ligand binding studies were combined to elucidate the expression of the alpha1-AR subtypes in various tissues of alpha1b +/+ and -/- mice. Total alpha1-AR sites were decreased by 98% in liver, 74% in heart, and 42% in cerebral cortex of the alpha1b -/- as compared with +/+ mice. Because of the large decrease of alpha1-AR in the heart and the loss of the alpha1b-AR mRNA in the aorta of the alpha1b-/- mice, the in vivo blood pressure and in vitro aorta contractile responses to alpha1-agonists were investigated in alpha1b +/+ and -/- mice. Our findings provide strong evidence that the alpha1b-AR is a mediator of the blood pressure and the aorta contractile responses induced by alpha1 agonists. This was demonstrated by the finding that the mean arterial blood pressure response to phenylephrine was decreased by 45% in alpha1b -/- as compared with +/+ mice. In addition, phenylephrine-induced contractions of aortic rings also were decreased by 25% in alpha1b-/- mice. The alpha1b-AR knockout mouse model provides a potentially useful tool to elucidate the functional specificity of different alpha1-AR subtypes, to better understand the effects of adrenergic drugs, and to investigate the multiple mechanisms involved in the control of blood pressure.
Figures

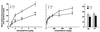
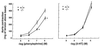
References
-
- Ruffolo R R, editor. The α1-Adrenergic Receptors. Clifton, NJ: Humana; 1987.
-
- Minneman K P, Esbenshade T A. Annu Rev Pharmacol Toxicol. 1994;34:117–133. - PubMed
-
- Michel M C, Kenny B, Schwinn D A. Naunyn-Schmiedeberg’s Arch Pharmacol. 1995;352:1–10. - PubMed
-
- Graham R M, Perez D M, Hwa J, Piascik M T. Circ Res. 1996;78:737–749. - PubMed
-
- Link R E, Stevens M S, Kulatunga M, Scheinin M, Barsh G S, Kobilka B K. Mol Pharmacol. 1995;48:48–55. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

