Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein
- PMID: 9326658
- PMCID: PMC23554
- DOI: 10.1073/pnas.94.21.11612
Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein
Abstract
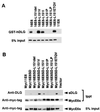
In the majority of cervical cancers, DNAs of high-risk mucosotpropic human papillomaviruses (HPVs), such as type 16, are maintained so as to express two viral proteins, E6 and E7, suggesting an essential importance to carcinogenesis. The high-risk HPV E6 proteins are known to inactivate p53 tumor suppressor protein but appear to have an additional, molecularly unknown function(s). In this study, we demonstrate that these E6 proteins can bind to the second PDZ domain of the human homologue of the Drosophila discs large tumor suppressor protein (hDLG) through their C-terminal XS/TXV/L (where X represents any amino acid, S/T serine or threonine, and V/L valine or leucine) motif. This finding is similar to the interaction between the adenomatous polyposis coli gene product and hDLG. E6 mutants losing the ability to bind to hDLG are no longer able to induce E6-dependent transformation of rodent cells. These results suggest an intriguing possibility that interaction between the E6 protein and hDLG or other PDZ domain-containing proteins could be an underlying mechanism in the development of HPV-associated cancers.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

