Ca2+-independent inhibition of inositol trisphosphate receptors by calmodulin: redistribution of calmodulin as a possible means of regulating Ca2+ mobilization
- PMID: 9326661
- PMCID: PMC23558
- DOI: 10.1073/pnas.94.21.11627
Ca2+-independent inhibition of inositol trisphosphate receptors by calmodulin: redistribution of calmodulin as a possible means of regulating Ca2+ mobilization
Abstract
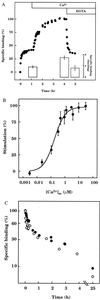
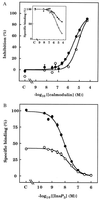
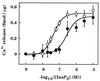
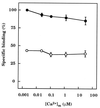
The interactions between calmodulin, inositol 1,4,5-trisphosphate (InsP3), and pure cerebellar InsP3 receptors were characterized by using a scintillation proximity assay. In the absence of Ca2+, 125I-labeled calmodulin reversibly bound to multiple sites on InsP3 receptors and Ca2+ increased the binding by 190% +/- 10%; the half-maximal effect occurred when the Ca2+ concentration was 184 +/- 14 nM. In the absence of Ca2+, calmodulin caused a reversible, concentration-dependent (IC50 = 3.1 +/- 0.2 microM) inhibition of [3H]InsP3 binding by decreasing the affinity of the receptor for InsP3. This effect was similar at all Ca2+ concentrations, indicating that the site through which calmodulin inhibits InsP3 binding has similar affinities for calmodulin and Ca2+-calmodulin. Calmodulin (10 microM) inhibited the Ca2+ release from cerebellar microsomes evoked by submaximal, but not by maximal, concentrations of InsP3. Tonic inhibition of InsP3 receptors by the high concentrations of calmodulin within cerebellar Purkinje cells may account for their relative insensitivity to InsP3 and limit spontaneous activation of InsP3 receptors in the dendritic spines. Inhibition of InsP3 receptors by calmodulin at all cytosolic Ca2+ concentrations, together with the known redistribution of neuronal calmodulin evoked by protein kinases and Ca2+, suggests that calmodulin may also allow both feedback control of InsP3 receptors and integration of inputs from other signaling pathways.
Figures

References
-
- Gnegy M E. Annu Rev Pharmacol Toxicol. 1993;33:45–70. - PubMed
-
- Carafoli E. J Biol Chem. 1992;267:2115–2118. - PubMed
-
- Buratti R, Prestipino G, Menegazzi P, Treves S, Zorzato F. Biochem Biophys Res Commun. 1995;213:1082–1090. - PubMed
-
- Liu M, Chen T-Y, Ahamed B, Li J, Yau K-W. Science. 1994;266:1348–1354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

