Decoding temporally encoded sensory input by cortical oscillations and thalamic phase comparators
- PMID: 9326662
- PMCID: PMC23560
- DOI: 10.1073/pnas.94.21.11633
Decoding temporally encoded sensory input by cortical oscillations and thalamic phase comparators
Abstract
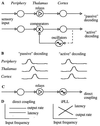
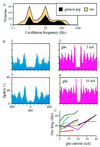
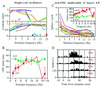
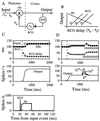
The temporally encoded information obtained by vibrissal touch could be decoded "passively," involving only input-driven elements, or "actively," utilizing intrinsically driven oscillators. A previous study suggested that the trigeminal somatosensory system of rats does not obey the bottom-up order of activation predicted by passive decoding. Thus, we have tested whether this system obeys the predictions of active decoding. We have studied cortical single units in the somatosensory cortices of anesthetized rats and guinea pigs and found that about a quarter of them exhibit clear spontaneous oscillations, many of them around whisking frequencies ( approximately 10 Hz). The frequencies of these oscillations could be controlled locally by glutamate. These oscillations could be forced to track the frequency of induced rhythmic whisker movements at a stable, frequency-dependent, phase difference. During these stimulations, the response intensities of multiunits at the thalamic recipient layers of the cortex decreased, and their latencies increased, with increasing input frequency. These observations are consistent with thalamocortical loops implementing phase-locked loops, circuits that are most efficient in decoding temporally encoded information like that obtained by active vibrissal touch. According to this model, and consistent with our results, populations of thalamic "relay" neurons function as phase "comparators" that compare cortical timing expectations with the actual input timing and represent the difference by their population output rate.
Figures

References
-
- Welker W I. Behavior. 1964;22:223–244.
-
- Gustafson J W, Felbain-Keramidas S L. Psychol Bull. 1977;84:477–488. - PubMed
-
- Simons D J. In: Cerebral Cortex. Jones E G, Diamond I T, editors; Jones E G, Diamond I T, editors. Vol. 11. New York: Plenum; 1995. pp. 263–297.
-
- Zucker E, Welker W I. Brain Res. 1969;12:138–156. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

