Activation of a caspase 3-related cysteine protease is required for glutamate-mediated apoptosis of cultured cerebellar granule neurons
- PMID: 9326666
- PMCID: PMC23571
- DOI: 10.1073/pnas.94.21.11657
Activation of a caspase 3-related cysteine protease is required for glutamate-mediated apoptosis of cultured cerebellar granule neurons
Abstract
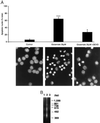
Neurotoxicity induced by overstimulation of N-methyl-D-aspartate (NMDA) receptors is due, in part, to a sustained rise in intracellular Ca2+; however, little is known about the ensuing intracellular events that ultimately result in cell death. Here we show that overstimulation of NMDA receptors by relatively low concentrations of glutamate induces apoptosis of cultured cerebellar granule neurons (CGNs) and that CGNs do not require new RNA or protein synthesis. Glutamate-induced apoptosis of CGNs is, however, associated with a concentration- and time-dependent activation of the interleukin 1beta-converting enzyme (ICE)/CED-3-related protease, CPP32/Yama/apopain (now designated caspase 3). Further, the time course of caspase 3 activation after glutamate exposure of CGNs parallels the development of apoptosis. Moreover, glutamate-induced apoptosis of CGNs is almost completely blocked by the selective cell permeable tetrapeptide inhibitor of caspase 3, Ac-DEVD-CHO but not by the ICE (caspase 1) inhibitor, Ac-YVAD-CHO. Western blots of cytosolic extracts from glutamate-exposed CGNs reveal both cleavage of the caspase 3 substrate, poly(ADP-ribose) polymerase, as well as proteolytic processing of pro-caspase 3 to active subunits. Our data demonstrate that glutamate-induced apoptosis of CGNs is mediated by a posttranslational activation of the ICE/CED-3-related cysteine protease caspase 3.
Figures




References
-
- Choi D W. Neuron. 1988;1:623–634. - PubMed
-
- Meldrum B, Garthwaite J. Trends Pharmacol Sci. 1990;11:379–387. - PubMed
-
- Coyle J T, Puttfarcken P. Science. 1993;262:689–695. - PubMed
-
- Lipton S A, Rosenberg P A. N Engl J Med. 1994;330:613–622. - PubMed
-
- Manev H, Favaron A, Guidotti A, Costa E. Mol Pharmacol. 1989;36:106–112. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

