The Drosophila neuregulin homolog Vein mediates inductive interactions between myotubes and their epidermal attachment cells
- PMID: 9334331
- PMCID: PMC316610
- DOI: 10.1101/gad.11.20.2691
The Drosophila neuregulin homolog Vein mediates inductive interactions between myotubes and their epidermal attachment cells
Abstract
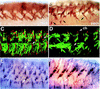
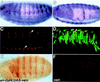
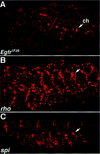
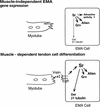
Inductive interactions between cells of distinct fates underlie the basis for morphogenesis and organogenesis across species. In the Drosophila embryo, somatic myotubes form specific interactions with their epidermal muscle attachment (EMA) cells. The establishment of these interactions is a first step toward further differentiation of the EMA cells into elongated tendon cells containing an organized array of microtubules and microfilaments. Here we show that the molecular signal for terminal differentiation of tendon cells is the secreted Drosophila neuregulin-like growth factor Vein produced by the myotubes. Although vein mRNA is produced by all of the myotubes, Vein protein is secreted and accumulates specifically at the muscle-tendon cell junctional site. In loss-of-function vein mutant embryos, muscle-dependent differentiation of tendon cells, measured by the level of expression of specific markers (Delilah and beta1 tubulin) is blocked. When Vein is expressed in ectopic ectodermal cells, it induces the ectopic expression of these genes. Our results favor the possibility that the Drosophila EGF receptor DER/Egfr expressed by the EMA cells functions as a receptor for Vein. We show that Vein/Egfr binding activates the Ras pathway in the EMA cells leading to the transcription of the tendon-specific genes, stripe, delilah, and beta1 tubulin. In Egfr1F26 mutant embryos that lack functional Egfr expression, the levels of Delilah and beta1 tubulin are very low. In addition, the ability of ectopic Vein to induce the expression of Delilah and beta1 tubulin depends on the presence of functional Egfrs. Finally, activation of the Egfr signaling pathway by either ectopically secreted Spitz, or activated Ras, leads to the ectopic expression of Delilah. These results suggest that inductive interactions between myotubes and their epidermal muscle attachment cells are initiated by the binding of Vein, to the Egfr on the surface of EMA cells.
Figures





References
-
- Abmayr SM, Erickson MS, Bour BA. Embryonic development of the larval body wall musculature of Drosophila melanogaster. Trends Genet. 1995;11:153–159. - PubMed
-
- Ashburner M. Drosophila: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. pp. 44–49.
-
- Azpiazu N, Lawrence PA, Vincent JP, Frasch M. Segmentation and specification of the Drosophila mesoderm. Genes & Dev. 1996;10:3183–3194. - PubMed
-
- Baker R, Schubiger G. Ectoderm induces muscle-specific gene expression in Drosophila embryos. Development. 1995;121:1387–1398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
