Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation
- PMID: 9334344
- PMCID: PMC2139803
- DOI: 10.1083/jcb.139.2.397
Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation
Abstract
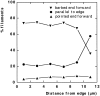
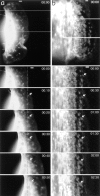
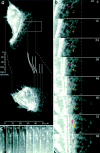
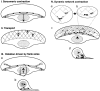
While the protrusive event of cell locomotion is thought to be driven by actin polymerization, the mechanism of forward translocation of the cell body is unclear. To elucidate the mechanism of cell body translocation, we analyzed the supramolecular organization of the actin-myosin II system and the dynamics of myosin II in fish epidermal keratocytes. In lamellipodia, long actin filaments formed dense networks with numerous free ends in a brushlike manner near the leading edge. Shorter actin filaments often formed T junctions with longer filaments in the brushlike area, suggesting that new filaments could be nucleated at sides of preexisting filaments or linked to them immediately after nucleation. The polarity of actin filaments was almost uniform, with barbed ends forward throughout most of the lamellipodia but mixed in arc-shaped filament bundles at the lamellipodial/cell body boundary. Myosin II formed discrete clusters of bipolar minifilaments in lamellipodia that increased in size and density towards the cell body boundary and colocalized with actin in boundary bundles. Time-lapse observation demonstrated that myosin clusters appeared in the lamellipodia and remained stationary with respect to the substratum in locomoting cells, but they exhibited retrograde flow in cells tethered in epithelioid colonies. Consequently, both in locomoting and stationary cells, myosin clusters approached the cell body boundary, where they became compressed and aligned, resulting in the formation of boundary bundles. In locomoting cells, the compression was associated with forward displacement of myosin features. These data are not consistent with either sarcomeric or polarized transport mechanisms of cell body translocation. We propose that the forward translocation of the cell body and retrograde flow in the lamellipodia are both driven by contraction of an actin-myosin network in the lamellipodial/cell body transition zone.
Figures







References
-
- Brands R, Feltkamp CA. Wet cleaving of cells: a method to introduce macromolecules into the cytoplasm. Application for immunolocalization of cytosol-exposed antigens. Exp Cell Res. 1988;176:309–318. - PubMed
-
- Byers, H.R., G.E. White, and K. Fujiwara. 1984. Organization and function of stress fibers in cells in vitro and in situ. In Cell and Muscle Motility. Vol. 5. J.W. Shay, editor. Plenum Publishing Corp., New York. 83–137. - PubMed

