The microtubule-dependent motor centromere-associated protein E (CENP-E) is an integral component of kinetochore corona fibers that link centromeres to spindle microtubules
- PMID: 9334346
- PMCID: PMC2139792
- DOI: 10.1083/jcb.139.2.435
The microtubule-dependent motor centromere-associated protein E (CENP-E) is an integral component of kinetochore corona fibers that link centromeres to spindle microtubules
Abstract
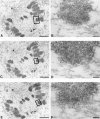
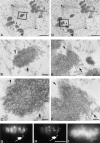
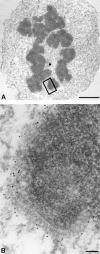
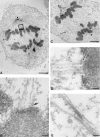
Centromere-associated protein E (CENP-E) is a kinesin-related microtubule motor protein that is essential for chromosome congression during mitosis. Using immunoelectron microscopy, CENP-E is shown to be an integral component of the kinetochore corona fibers that tether centromeres to the spindle. Immediately upon nuclear envelope fragmentation, an associated plus end motor trafficks cytoplasmic CENP-E toward chromosomes along astral microtubules that enter the nuclear volume. Before or concurrently with initial lateral attachment of spindle microtubules, CENP-E targets to the outermost region of the developing kinetochores. After stable attachment, throughout chromosome congression, at metaphase, and throughout anaphase A, CENP-E is a constituent of the corona fibers, extending at least 50 nm away from the kinetochore outer plate and intertwining with spindle microtubules. In congressing chromosomes, CENP-E is preferentially associated with (or accessible at) the stretched, leading kinetochore known to provide the primary power for chromosome movement. Taken together, this evidence strongly supports a model in which CENP-E functions in congression to tether kinetochores to the disassembling microtubule plus ends.
Figures






Similar articles
-
Localization of CENP-E in the fibrous corona and outer plate of mammalian kinetochores from prometaphase through anaphase.Chromosoma. 1997 Dec;106(7):446-55. doi: 10.1007/s004120050266. Chromosoma. 1997. PMID: 9391217
-
Kinetochore kinesin CENP-E is a processive bi-directional tracker of dynamic microtubule tips.Nat Cell Biol. 2013 Sep;15(9):1079-1088. doi: 10.1038/ncb2831. Epub 2013 Aug 18. Nat Cell Biol. 2013. PMID: 23955301 Free PMC article.
-
Mechanisms of kinesin-7 CENP-E in kinetochore-microtubule capture and chromosome alignment during cell division.Biol Cell. 2019 Jun;111(6):143-160. doi: 10.1111/boc.201800082. Epub 2019 Feb 26. Biol Cell. 2019. PMID: 30784092 Review.
-
A conserved CENP-E region mediates BubR1-independent recruitment to the outer corona at mitotic onset.Curr Biol. 2024 Mar 11;34(5):1133-1141.e4. doi: 10.1016/j.cub.2024.01.042. Epub 2024 Feb 13. Curr Biol. 2024. PMID: 38354735 Free PMC article.
-
Leaving no-one behind: how CENP-E facilitates chromosome alignment.Essays Biochem. 2020 Sep 4;64(2):313-324. doi: 10.1042/EBC20190073. Essays Biochem. 2020. PMID: 32347304 Free PMC article. Review.
Cited by
-
A mitotic septin scaffold required for Mammalian chromosome congression and segregation.Science. 2005 Mar 18;307(5716):1781-5. doi: 10.1126/science.1106823. Science. 2005. PMID: 15774761 Free PMC article.
-
Potential of Farnesyl Transferase Inhibitors in Combination Regimens in Squamous Cell Carcinomas.Cancers (Basel). 2021 Oct 22;13(21):5310. doi: 10.3390/cancers13215310. Cancers (Basel). 2021. PMID: 34771475 Free PMC article.
-
Dynein light intermediate chain: an essential subunit that contributes to spindle checkpoint inactivation.Mol Biol Cell. 2008 Nov;19(11):4918-29. doi: 10.1091/mbc.e08-05-0483. Epub 2008 Sep 17. Mol Biol Cell. 2008. PMID: 18799620 Free PMC article.
-
Phosphorylation of HsMis13 by Aurora B kinase is essential for assembly of functional kinetochore.J Biol Chem. 2008 Sep 26;283(39):26726-36. doi: 10.1074/jbc.M804207200. Epub 2008 Jul 17. J Biol Chem. 2008. PMID: 18640974 Free PMC article.
-
Syntelin inhibits triple-negative breast cancer cell proliferation and metastasis.J Mol Cell Biol. 2022 Jan 21;13(11):834-837. doi: 10.1093/jmcb/mjab054. J Mol Cell Biol. 2022. PMID: 34450654 Free PMC article. No abstract available.
References
-
- Brinkley BR, Stubblefield E. The fine structure of the kinetochore of a mammalian cell in vitro. . Chromosoma (Berl) 1966;19:28–43. - PubMed
-
- Brown KD, Wood KW, Cleveland DW. The kinesin-like protein CENP-E is kinetochore-associated thoughout poleward chromosome segregation during anaphase-A. J Cell Sci. 1996;109:961–969. - PubMed
-
- Chen R-H, Waters JC, Salmon ED, Murray AW. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science (Wash DC) 1996;274:242–246. - PubMed
-
- Comings DE, Okada TA. Holocentric chromosomes in oncopettus kinetochore plates are present in mitosis but absent in meiosis. Chromosoma (Berl) 1973;37:177–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

