The yeast motor protein, Kar3p, is essential for meiosis I
- PMID: 9334348
- PMCID: PMC2139793
- DOI: 10.1083/jcb.139.2.459
The yeast motor protein, Kar3p, is essential for meiosis I
Abstract
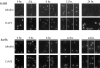
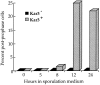
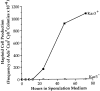
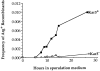
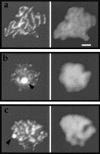
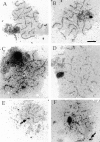
The recognition and alignment of homologous chromosomes early in meiosis is essential for their subsequent segregation at anaphase I; however, the mechanism by which this occurs is unknown. We demonstrate here that, in the absence of the molecular motor, Kar3p, meiotic cells are blocked with prophase monopolar microtubule arrays and incomplete synaptonemal complex (SC) formation. kar3 mutants exhibit very low levels of heteroallelic recombination. kar3 mutants do produce double-strand breaks that act as initiation sites for meiotic recombination in yeast, but at levels severalfold reduced from wild-type. These data are consistent with a meiotic role for Kar3p in the events that culminate in synapsis of homologues.
Figures

References
-
- Afshar K, Barton NR, Hawley RS, Goldstein LSB. DNA binding and meiotic chromosomal localization of the Drosophila nod kinesin-like protein. Cell. 1995;81:129–138. - PubMed
-
- Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. - PubMed
-
- Bascom-Slack, C.A., L.O. Ross, and D.S. Dawson. 1997. Chiasmata, crossovers, and meiotic chromosome segregation. In Advances in Genetics. Vol. 35. J. Hall and J. Dunlap, editors. Academic Press, Inc., San Diego, CA. 253–284. - PubMed
-
- Bishop DK, Park D, Xu L, Kleckner N. DMC1: a meiosis-specific yeast homolog of E. coli recArequired for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992;69:439–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

