ATP- and gap junction-dependent intercellular calcium signaling in osteoblastic cells
- PMID: 9334351
- PMCID: PMC2139805
- DOI: 10.1083/jcb.139.2.497
ATP- and gap junction-dependent intercellular calcium signaling in osteoblastic cells
Abstract
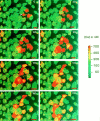
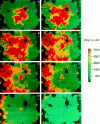
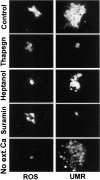
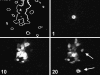
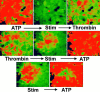
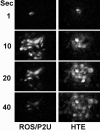
Many cells coordinate their activities by transmitting rises in intracellular calcium from cell to cell. In nonexcitable cells, there are currently two models for intercellular calcium wave propagation, both of which involve release of inositol trisphosphate (IP3)- sensitive intracellular calcium stores. In one model, IP3 traverses gap junctions and initiates the release of intracellular calcium stores in neighboring cells. Alternatively, calcium waves may be mediated not by gap junctional communication, but rather by autocrine activity of secreted ATP on P2 purinergic receptors. We studied mechanically induced calcium waves in two rat osteosarcoma cell lines that differ in the gap junction proteins they express, in their ability to pass microinjected dye from cell to cell, and in their expression of P2Y2 (P2U) purinergic receptors. ROS 17/2.8 cells, which express the gap junction protein connexin43 (Cx43), are well dye coupled, and lack P2U receptors, transmitted slow gap junction-dependent calcium waves that did not require release of intracellular calcium stores. UMR 106-01 cells predominantly express the gap junction protein connexin 45 (Cx45), are poorly dye coupled, and express P2U receptors; they propagated fast calcium waves that required release of intracellular calcium stores and activation of P2U purinergic receptors, but not gap junctional communication. ROS/P2U transfectants and UMR/Cx43 transfectants expressed both types of calcium waves. Gap junction-independent, ATP-dependent intercellular calcium waves were also seen in hamster tracheal epithelia cells. These studies demonstrate that activation of P2U purinergic receptors can propagate intercellular calcium, and describe a novel Cx43-dependent mechanism for calcium wave propagation that does not require release of intracellular calcium stores by IP3. These studies suggest that gap junction communication mediated by either Cx43 or Cx45 does not allow passage of IP3 well enough to elicit release of intracellular calcium stores in neighboring cells.
Figures


References
-
- Babich M, Choi H, Johnson RM, King KL, Alford GE, Nissenson RA. Thrombin and parathyroid hormone mobilize intracellular calcium in rat osteosarcoma cells by distinct pathways. Endocrinology. 1991;129:1463–1470. - PubMed
-
- Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science (Wash DC) 1992;258:292–295. - PubMed
-
- Cao D, Lin G, Westphale EM, Beyer EC, Steinberg TH. Mechanisms for the coordination of intercellular calcium signaling in insulin-secreting cells. J Cell Sci. 1997;110:497–504. - PubMed
-
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

