Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis
- PMID: 9334355
- PMCID: PMC2139797
- DOI: 10.1083/jcb.139.2.541
Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis
Abstract
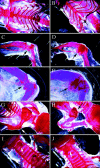
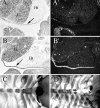
Members of the TGF-beta superfamily are important regulators of skeletal development. TGF-betas signal through heteromeric type I and type II receptor serine/threonine kinases. When over-expressed, a cytoplasmically truncated type II receptor can compete with the endogenous receptors for complex formation, thereby acting as a dominant-negative mutant (DNIIR). To determine the role of TGF-betas in the development and maintenance of the skeleton, we have generated transgenic mice (MT-DNIIR-4 and -27) that express the DNIIR in skeletal tissue. DNIIR mRNA expression was localized to the periosteum/perichondrium, syno-vium, and articular cartilage. Lower levels of DNIIR mRNA were detected in growth plate cartilage. Transgenic mice frequently showed bifurcation of the xiphoid process and sternum. They also developed progressive skeletal degeneration, resulting by 4 to 8 mo of age in kyphoscoliosis and stiff and torqued joints. The histology of affected joints strongly resembled human osteo-arthritis. The articular surface was replaced by bone or hypertrophic cartilage as judged by the expression of type X collagen, a marker of hypertrophic cartilage normally absent from articular cartilage. The synovium was hyperplastic, and cartilaginous metaplasia was observed in the joint space. We then tested the hypothesis that TGF-beta is required for normal differentiation of cartilage in vivo. By 4 and 8 wk of age, the level of type X collagen was increased in growth plate cartilage of transgenic mice relative to wild-type controls. Less proteoglycan staining was detected in the growth plate and articular cartilage matrix of transgenic mice. Mice that express DNIIR in skeletal tissue also demonstrated increased Indian hedgehog (IHH) expression. IHH is a secreted protein that is expressed in chondrocytes that are committed to becoming hypertrophic. It is thought to be involved in a feedback loop that signals through the periosteum/ perichondrium to inhibit cartilage differentiation. The data suggest that TGF-beta may be critical for multifaceted maintenance of synovial joints. Loss of responsiveness to TGF-beta promotes chondrocyte terminal differentiation and results in development of degenerative joint disease resembling osteoarthritis in humans.
Figures







References
-
- Ballock RT, Heydemann A, Wakefield LM, Flanders KC, Roberts AB, Sporn MB. TGF-β1 prevents hypertrophy of epiphyseal chondrocytes: regulation of gene expression for cartilage matrix proteins and metalloproteases. Dev Biol. 1993;158:414–429. - PubMed
-
- Bohme K, Winterhalter KH, Bruckner P. Terminal differentiation of chondrocytes in culture is a spontaneous process and is arrested by TGF-β2 and basic fibroblast growth factor in synergy. Exp Cell Res. 1995;216:191–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

