Presentation of integrins on leukocyte microvilli: a role for the extracellular domain in determining membrane localization
- PMID: 9334357
- PMCID: PMC2139798
- DOI: 10.1083/jcb.139.2.563
Presentation of integrins on leukocyte microvilli: a role for the extracellular domain in determining membrane localization
Abstract
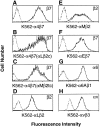
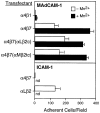
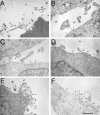
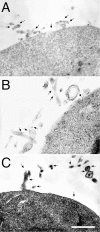
Adhesion of blood leukocytes to the endothelium involves multiple steps including initial attachment (tethering), rolling, and firm arrest. Presentation of adhesion molecules on leukocyte microvilli can substantially enhance tethering. Localization of L-selectin to microvilli and of CD44 to the planar cell body have been shown to depend upon their transmembrane and cytoplasmic domains. We investigated the role of leukocyte integrin transmembrane and cytoplasmic domains in initiating adhesion under flow and in microvillous localization. Integrins alpha4beta7, alphaLbeta2, and alphaMbeta2 were heterologously expressed in K562 cells. alpha4beta7 initiated adhesion under flow and localized to microvilli, whereas beta2 integrins did not initiate adhesion and localized to the cell body. Chimeric integrins were produced by replacing the alpha4beta7 cytoplasmic and/or transmembrane domains with the homologous domains of alphaLbeta2 or alphaMbeta2. Unexpectedly, these chimeras efficiently mediated adhesion to the alpha4beta7 ligand mucosal addressin cell adhesion molecule-1 under flow and localized to microvilli. Therefore, differences between the transmembrane and cytoplasmic domains of alpha4 and beta2 integrins do not account for differences in ability to support attachment under flow or in membrane localization. Integrins alpha4beta1, alpha5beta1, alpha6Abeta1, alphavbeta3, and alphaEbeta7 also localized to microvilli. Transmembrane proteins known or suspected to associate with extracellular domains of microvillous integrins, including tetraspans and CD47, were concentrated on microvilli as well. These findings suggest that interactions between the extracellular domains of integrins and associated proteins could direct the assembly of multimolecular complexes on leukocyte microvilli.
Figures


References
-
- Andrew DP, Berlin C, Honda S, Yoshino T, Hamann A, Holzmann B, Kilshaw PJ, Butcher EC. Distinct but overlapping epitopes are involved in α4β7-mediated adhesion to vascular cell adhesion molecule-1, mucosal addressin-1, fibronectin, and lymphocyte aggregation. J Immunol. 1994;153:3847–3861. - PubMed
-
- Berditchevski F, Bazzoni G, Hemler ME. Specific association of CD63 with the VLA-3 and VLA-6 integrins. J Biol Chem. 1995;270:17784–17790. - PubMed
-
- Berditchevski F, Tolias KF, Wong K, Carpenter CL, Hemler ME. A novel link between integrins, transmembrane-4 superfamily proteins (CD63 and CD81), and phosphatidylinositol 4-kinase. J Biol Chem. 1997;272:2595–2598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

