Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity
- PMID: 9334364
- PMCID: PMC2199096
- DOI: 10.1084/jem.186.8.1247
Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity
Abstract
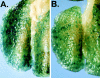
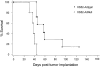
Dendritic cells (DCs) are potent antigen-presenting cells that play a critical role in the initiation of antitumor immune responses. In this study, we show that genetic modifications of a murine epidermis-derived DC line and primary bone marrow-derived DCs to express a model antigen beta-galactosidase (betagal) can be achieved through the use of a replication-deficient, recombinant adenovirus vector, and that the modified DCs are capable of eliciting antigen-specific, MHC-restricted CTL responses. Importantly, using a murine metastatic lung tumor model with syngeneic colon carcinoma cells expressing betagal, we show that immunization of mice with the genetically modified DC line or bone marrow DCs confers potent protection against a lethal tumor challenge, as well as suppression of preestablished tumors, resulting in a significant survival advantage. We conclude that genetic modification of DCs to express antigens that are also expressed in tumors can lead to antigen-specific, antitumor killer cells, with a concomitant resistance to tumor challenge and a decrease in the size of existing tumors.
Figures
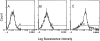
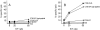









References
-
- Pardoll DM. Cancer vaccines. Immunol Today. 1993;14:310–316. - PubMed
-
- Juillard V, Villefroy P, Godfrin D, Pavirani A, Venet A, Guillet JG. Long-term humoral and cellular immunity induced by a single immunization with replication-defective adenovirus recombinant vector. Eur J Immunol. 1995;25:3467–3473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

