The common cytokine receptor gamma chain controls survival of gamma/delta T cells
- PMID: 9334367
- PMCID: PMC2199080
- DOI: 10.1084/jem.186.8.1277
The common cytokine receptor gamma chain controls survival of gamma/delta T cells
Abstract
We have investigated the role of common gamma chain (gamma c)-signaling pathways for the development of T cell receptor for antigen (TCR)-gamma/delta T cells. TCR-gamma/delta-bearing cells were absent from the adult thymus, spleen, and skin of gamma c-deficient (gamma c-) mice, whereas small numbers of thymocytes expressing low levels of TCR-gamma/delta were detected during fetal life. Recent reports have suggested that signaling via interleukin (IL)-7 plays a major role in facilitating TCR-gamma/delta development through induction of V-J (variable-joining) rearrangements at the TCR-gamma locus. In contrast, we detected clearly TCR-gamma rearrangements in fetal thymi from gamma c- mice (which fail to signal in response to IL-7) and reduced TCR-gamma rearrangements in adult gamma c thymi. No gross defects in TCR-delta or TCR-beta rearrangements were observed in gamma c- mice of any age. Introduction of productively rearranged TCR V gamma 1 or TCR V gamma 1/V delta 6 transgenes onto mice bearing the gamma c mutation did not restore TCR-gamma/delta development to normal levels suggesting that gamma c-dependent pathways provide additional signals to developing gamma/delta T cells other than for the recombination process. Bcl-2 levels in transgenic thymocytes from gamma c- mice were dramatically reduced compared to gamma c+ transgenic littermates. We favor the concept that gamma c-dependent receptors are required for the maintenance of TCR-gamma/delta cells and contribute to the completion of TCR-gamma rearrangements primarily by promoting survival of cells committed to the TCR-gamma/delta lineage.
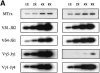
Figures








References
-
- Kisielow P, von Boehmer H. Development and selection of T cells: facts and puzzles. Adv Immunol. 1995;58:87–209. - PubMed
-
- Allison JP. γδ T-cell development. Curr Opin Immunol. 1993;5:241–246. - PubMed
-
- Hass W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. - PubMed
-
- Lafaille JJ, DeCloux A, Bonneville M, Takagaki Y, Tonegawa S. Junctional sequences of T cell receptor γδ genes: implications for γδ T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989;59:859–870. - PubMed
-
- Bogue M, Mossman H, Stauffer U, Benoist C, Mathis D. The level of N-region diversity in T cell receptors is not pre-ordained in the stem cell. Eur J Immunol. 1993;23:1185–1188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

