Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding
- PMID: 9334377
- PMCID: PMC2199098
- DOI: 10.1084/jem.186.8.1373
Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding
Abstract
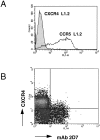
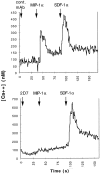
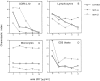
CCR5 is a chemokine receptor expressed by T cells and macrophages, which also functions as the principal coreceptor for macrophage (M)-tropic strains of HIV-1. To understand the molecular basis of the binding of chemokines and HIV-1 to CCR5, we developed a number of mAbs that inhibit the various interactions of CCR5, and mapped the binding sites of these mAbs using a panel of CCR5/CCR2b chimeras. One mAb termed 2D7 completely blocked the binding and chemotaxis of the three natural chemokine ligands of CCR5, RANTES (regulated on activation normal T cell expressed and secreted), macrophage inflammatory protein (MIP)-1alpha, and MIP-1beta, to CCR5 transfectants. This mAb was a genuine antagonist of CCR5, since it failed to stimulate an increase in intracellular calcium concentration in the CCR5 transfectants, but blocked calcium responses elicited by RANTES, MIP-1alpha, or MIP-1beta. This mAb inhibited most of the RANTES and MIP-1alpha chemotactic responses of activated T cells, but not of monocytes, suggesting differential usage of chemokine receptors by these two cell types. The 2D7 binding site mapped to the second extracellular loop of CCR5, whereas a group of mAbs that failed to block chemokine binding all mapped to the NH2-terminal region of CCR5. Efficient inhibition of an M-tropic HIV-1-derived envelope glycoprotein gp120 binding to CCR5 could be achieved with mAbs recognizing either the second extracellular loop or the NH2-terminal region, although the former showed superior inhibition. Additionally, 2D7 efficiently blocked the infectivity of several M-tropic and dual-tropic HIV-1 strains in vitro. These results suggest a complicated pattern of HIV-1 gp120 binding to different regions of CCR5, but a relatively simple pattern for chemokine binding. We conclude that the second extracellular loop of CCR5 is an ideal target site for the development of inhibitors of either chemokine or HIV-1 binding to CCR5.
Figures

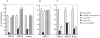

References
-
- Baggiolini M, Dewald B, Moser B. IL-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol. 1994;55:97–179. - PubMed
-
- Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617–648. - PubMed
-
- Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12:17–46. - PubMed
-
- Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo J-A, Newman W, et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties provide a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–612. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

