Angiotensin II AT1A receptor mRNA expression is induced by estrogen-progesterone in dopaminergic neurons of the female rat arcuate nucleus
- PMID: 9334403
- PMCID: PMC6573735
- DOI: 10.1523/JNEUROSCI.17-21-08283.1997
Angiotensin II AT1A receptor mRNA expression is induced by estrogen-progesterone in dopaminergic neurons of the female rat arcuate nucleus
Abstract
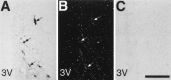
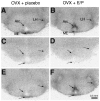
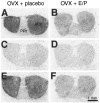
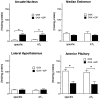
Brain angiotensin II (Ang II) inhibits pituitary prolactin release by an indirect mechanism requiring stimulation of dopamine formation and release. We report that [125I]Sar1-Ang II binding to AT1 receptors and AT1A receptor mRNA expression increase selectively in the dorsomedial arcuate nucleus of 17beta-estradiol-primed ovariectomized rats after treatment with progesterone. In hormone-treated rats, arcuate nucleus AT1A receptor mRNA expression is associated with tyrosine hydroxylase-positive neurons. No AT1A receptor mRNA was detected in tyrosine hydroxylase-positive cells of the arcuate nucleus of intact male rats. Conversely, in the anterior pituitary, where local or circulating Ang II stimulates prolactin release, [125I]Sar1-Ang II binding to AT1 receptors and AT1B receptor mRNA expression are decreased in 17beta-estradiol/progesterone-treated ovariectomized rats. Thus, AT1A receptors in the dorsal arcuate nucleus and AT1B receptors in the anterior pituitary are regulated inversely by estrogen/progesterone treatment, supporting the hypothesis of a dual role for brain and pituitary Ang II on prolactin release. The colocalization of AT1A receptor mRNA and tyrosine hydroxylase in neurons of the arcuate nucleus furthermore indicates that within this area central Ang II acts directly on dopaminergic neurons. These results support the hypothesis that central Ang II inhibits pituitary prolactin release indirectly via modulation of dopaminergic activity in the arcuate nucleus.
Figures


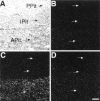
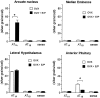

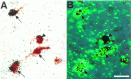
References
-
- Aguilera G, Hyde C, Catt KJ. Angiotensin II receptors and prolactin release in pituitary lactotrophs. Endocrinology. 1982;111:1045–1050. - PubMed
-
- Arbogast LA, Voogt JL. Progesterone reverses the estradiol-induced decrease in tyrosine hydroxylase mRNA levels in the arcuate nucleus. Neuroendocrinology. 1993;58:501–510. - PubMed
-
- Barron WM, Schreiber J, Lindheimer MD. Effect of ovarian sex steroids on osmoregulation and vasopressin secretion in the rat. Am J Physiol. 1986;250:E352–E361. - PubMed
-
- Becú-Villalobos D, Lacau-Mengido IM, Thyssen SM, Díaz-Torga GS, Libertun C. Effects of LHRH and Ang II on prolactin stimulation are mediated by hypophysial AT1 receptor subtype. Am J Physiol. 1994;266:E274–E278. - PubMed
-
- Ben-Jonathan N, Oliver C, Weiner HJ, Mical RS, Porter JC. Dopamine in hypophysial portal plasma of the rat during the estrous cycle and throughout pregnancy. Endocrinology. 1977;100:452–458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
