Expression of neural RNA-binding proteins in the postnatal CNS: implications of their roles in neuronal and glial cell development
- PMID: 9334405
- PMCID: PMC6573750
- DOI: 10.1523/JNEUROSCI.17-21-08300.1997
Expression of neural RNA-binding proteins in the postnatal CNS: implications of their roles in neuronal and glial cell development
Abstract
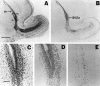
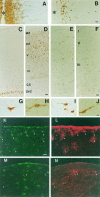
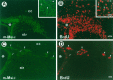
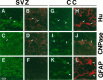
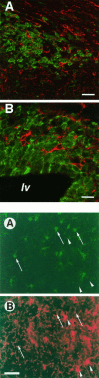
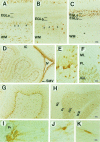
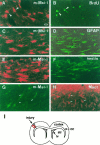
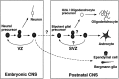
There is an increasing interest in the role of RNA-binding proteins during neural development. Mouse-Musashi-1 (m-Msi-1) is a mouse neural RNA-binding protein with sequence similarity to Drosophila musashi (d-msi), which is essential for neural development. m-Msi-1 is highly enriched in neural precursor cells that are capable of generating both neurons and glia during embryonic CNS development. The present study characterized m-Msi-1-expressing cells in the postnatal and adult CNS. Postnatally, m-Msi-1 was expressed in proliferative neuronal precursors in the external granule cell layer of the cerebellum and in the anterior corner of the subventricular zone of the lateral ventricles. In gliogenesis, the persistent expression of m-Msi-1 was observed in cells of the astrocyte lineage ranging from proliferative glial precursors in the subventricular zone (SVZ) to differentiated astrocytes in the parenchyma. In addition, we showed that m-Msi-1 was still expressed in proliferating cells in the adult SVZ, which may contain neural precursor or stem cells. Another neural RNA-binding protein Hu (the mammalian homolog of a Drosophila neuronal RNA-binding protein Elav) was present in postmitotic neurons throughout the development of the CNS, and its pattern of expression was compared with that of m-Msi-1. These observations imply that these two RNA-binding proteins may be involved in the development of neurons and glia by regulating gene expression at the post-transcriptional level.
Figures
References
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. J Comp Neurol. 1972;145:353–398. - PubMed
-
- Bignami A, Dahl D. Astrocyte-specific protein and neuroglial differentiation. An immunofluorescence study with antibodies to the glial fibrillary acidic protein. J Comp Neurol. 1974;153:27–38. - PubMed
-
- Bignami A, Dahl D. The astroglial response to stabbing. Immunofluorescence studies with antibodies to astrocyte-specific protein (GFA) in mammalian and submammalian vertebrates. Neuropathol Appl Neurobiol. 1976;2:99–110.
-
- Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–641. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
