Transition from growth cone to functional motor nerve terminal in Drosophila embryos
- PMID: 9334414
- PMCID: PMC6573746
- DOI: 10.1523/JNEUROSCI.17-21-08408.1997
Transition from growth cone to functional motor nerve terminal in Drosophila embryos
Abstract
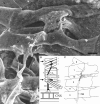
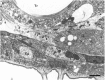
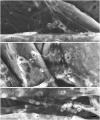
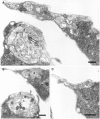
As a motor axon grows from the CNS to its target muscle, the terminal has the form of a flattened growth cone with a planar central region, lamellipodia, and filopodia. A mature terminal usually has a stereotyped shape that may be elongated with varicosities, as in several invertebrate species, or have short branches with boutons, as in mammals. We examined in Drosophila the developmental changes between growth cone and mature terminal using ultrastructural and immunocytochemical methods. The transition period, which occurs 2-3 hr after the first growth cone reaches its target muscle, is marked by the formation of "prevaricosities," smoothly contoured enlargements of the axons at the point where the nerve trunk first contacts the muscle fiber (MF). There is a 15-30 min ventral-to-dorsal gradient in the formation of prevaricosities on the individual abdominal MFs. Multineuronal innervation of each MF has occurred by this time, and two or more different axons undergo prevaricosity formation while they are intimately intertwined at the nerve entry point (NEP). Presynaptic active zones, both nerve-nerve and nerve-muscle, occur within the prevaricosities along broad contact regions. Synaptotagmin immunoreactive clusters form concurrently. The first varicosities then develop as a result of constrictions of the larger prevaricosities rather than as enlargement of discrete portions of the filopodia or neurites. The prevaricosity stage therefore may include the key steps that lead to the differentiation of functional differences in terminal subtypes as well as those leading to the formation of a stable neuromuscular junction.
Figures










References
-
- Ashburner M. Drosophila: a laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989.
-
- Atwood HL, Wojtowicz JM. Short-term and long-term plasticity and physiological differentiation of crustacean motor synapses. Int Rev Neurobiol. 1986;28:275–362. - PubMed
-
- Atwood HL, Govind CK, Wu C-F. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–1024. - PubMed
-
- Bate M. The embryonic development of larval muscles in Drosophila. Development. 1990;110:791–804. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
