Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance
- PMID: 9334425
- PMCID: PMC6573725
- DOI: 10.1523/JNEUROSCI.17-21-08528.1997
Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance
Abstract
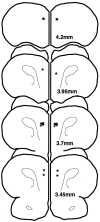
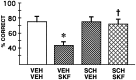
Although previous research has emphasized the beneficial effects of dopamine (DA) on functions of the prefrontal cortex (PFC), recent studies of animals exposed to mild stress indicate that excessive DA receptor stimulation may be detrimental to the spatial working memory functions of the PFC (Arnsten and Goldman-Rakic, 1990; Murphy et al., 1994, 1996a,b, 1997). In particular, these studies have suggested that supranormal stimulation of D1 receptors may contribute to the detrimental actions of DA in the PFC (Murphy et al., 1994, 1996a). The current study directly tested this hypothesis by examining the effects of infusing a full D1 receptor agonist, SKF 81297, into the PFC of rats performing a spatial working memory task, delayed alternation. SKF 81297 produced a dose-related impairment in delayed-alternation performance. The impairment was reversed by pretreatment with a D1 receptor antagonist, SCH 23390, consistent with drug actions at D1 receptors. SCH 23390 by itself had no effect on performance, although slightly higher doses impaired performance (Murphy et al., 1994, 1996a). There was a significant relationship between infusion location and drug efficacy; animals with cannulae anterior to the PFC were not impaired by SKF 81297 infusions. Taken together, these results demonstrate that supranormal D1 receptor stimulation in the PFC is sufficient to impair PFC working memory function. These cognitive data are consistent with recent electrophysiological studies of D1 receptor mechanisms affecting the PFC (Williams and Goldman-Rakic, 1995; Yang and Seamans, 1996). Increased D1 receptor stimulation during stress may serve to take the PFC "off-line" to allow posterior cortical and subcortical structures to regulate behavior, but may contribute to the vulnerability of the PFC in many neuropsychiatric disorders.
Figures




References
-
- Andersen PH, Jansen JA. Dopamine receptor agonists: selectivity and D1 receptor efficacy. Eur J Pharmacol. 1990;188:335–347. - PubMed
-
- Arnsten AFT. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–162. - PubMed
-
- Arnsten AFT, Goldman-Rakic PS. Reversal of stress-induced delayed response deficits in rhesus monkeys by clonidine and naloxone. Soc Neurosci Abstr. 1986;12:1464.
-
- Arnsten AFT, Goldman-Rakic PS. Stress impairs prefrontal cortex cognitive function in monkeys: role of dopamine. Soc Neurosci Abstr. 1990;16:164.
-
- Arnsten AFT, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
