Systemic morphine-induced Fos protein in the rat striatum and nucleus accumbens is regulated by mu opioid receptors in the substantia nigra and ventral tegmental area
- PMID: 9334431
- PMCID: PMC6573751
- DOI: 10.1523/JNEUROSCI.17-21-08596.1997
Systemic morphine-induced Fos protein in the rat striatum and nucleus accumbens is regulated by mu opioid receptors in the substantia nigra and ventral tegmental area
Abstract
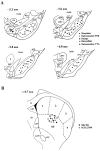
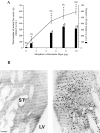
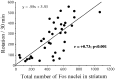
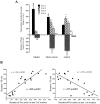
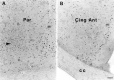
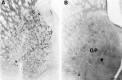
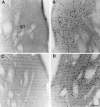
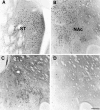
To characterize how systemic morphine induces Fos protein in dorsomedial striatum and nucleus accumbens (NAc), we examined the role of receptors in striatum, substantia nigra (SN), and ventral tegmental area (VTA). Morphine injected into medial SN or into VTA of awake rats induced Fos in neurons in ipsilateral dorsomedial striatum and NAc. Morphine injected into lateral SN induced Fos in dorsolateral striatum and globus pallidus. The morphine infusions produced contralateral turning that was most prominent after lateral SN injections. Intranigral injections of [D-Ala2, N-Me-Phe4, Gly-ol5]-enkephalin (DAMGO), a mu opioid receptor agonist, and of bicuculline, a GABAA receptor antagonist, induced Fos in ipsilateral striatum. Fos induction in dorsomedial striatum produced by systemic administration of morphine was blocked by (1) SN and VTA injections of the mu1 opioid antagonist naloxonazine and (2) striatal injections of either MK 801, an NMDA glutamate receptor antagonist, or SCH 23390, a D1 dopamine receptor antagonist. Fos induction in dorsomedial striatum and NAc after systemic administration of morphine seems to be mediated by dopamine neurons in medial SN and VTA that project to medial striatum and NAc, respectively. Systemic morphine is proposed to act on mu opioid receptors located on GABAergic interneurons in medial SN and VTA. Inhibition of these GABA interneurons disinhibits medial SN and VTA dopamine neurons, producing dopamine release in medial striatum and NAc. This activates D1 dopamine receptors and coupled with the coactivation of NMDA receptors possibly from cortical glutamate input induces Fos in striatal and NAc neurons. The modulation of target gene expression by Fos could influence addictive behavioral responses to opiates.
Figures





References
-
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223–255. - PubMed
-
- Albin RL, Young AB, Penney JB. The functional anatomy of disorders of the basal ganglia. Trends Neurosci. 1995;18:63–64. - PubMed
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
-
- Aronin N, Chase K, Sagar SM, Sharp FR, DiFiglia M. N-methyl-d-aspartate receptor activation in the neostriatum increases c-fos and fos-related antigens selectively in medium-sized neurons. Neuroscience. 1991;44:409–420. - PubMed
-
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signalling pathways. Science. 1993;260:181–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
