Protein-protein interactions among the Aux/IAA proteins
- PMID: 9342315
- PMCID: PMC23574
- DOI: 10.1073/pnas.94.22.11786
Protein-protein interactions among the Aux/IAA proteins
Abstract
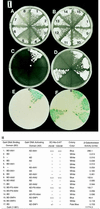
The plant hormone indoleacetic acid (IAA) transcriptionally activates early genes in plants. The Aux/IAA family of early genes encodes proteins that are short-lived and nuclear-localized. They also contain a putative prokaryotic betaalphaalpha DNA binding motif whose formation requires protein dimerization. Here, we show that the pea PS-IAA4 and Arabidopsis IAA1 and IAA2 proteins perform homo- and heterotypic interactions in yeast using the two-hybrid system. Gel-filtration chromatography and chemical cross-linking experiments demonstrate that the PS-IAA4 and IAA1 proteins interact to form homodimers in vitro. Deletion analysis of PS-IAA4 indicates that the betaalphaalpha containing acidic C terminus of the protein is necessary for homotypic interactions in the yeast two-hybrid system. Screening an Arabidopsis lambda-ACT cDNA library using IAA1 as a bait reveals heterotypic interactions of IAA1 with known and newly discovered members of the Arabidopsis Aux/IAA gene family. The new member IAA24 has similarity to ARF1, a transcription factor that binds to an auxin response element. Combinatorial interactions among the various members of the Aux/IAA gene family may regulate a variety of late genes as well as serve as autoregulators of early auxin-regulated gene expression. These interactions provide a molecular basis for the developmental and tissue-specific manner of auxin action.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

