Structural evidence for a second sialic acid binding site in avian influenza virus neuraminidases
- PMID: 9342319
- PMCID: PMC23599
- DOI: 10.1073/pnas.94.22.11808
Structural evidence for a second sialic acid binding site in avian influenza virus neuraminidases
Abstract
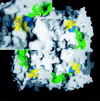
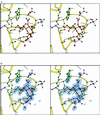
The x-ray structure of a complex of sialic acid (Neu5Ac) with neuraminidase N9 subtype from A/tern/Australia/G70C/75 influenza virus at 4 degrees C has revealed the location of a second Neu5Ac binding site on the surface of the enzyme. At 18 degrees C, only the enzyme active site contains bound Neu5Ac. Neu5Ac binds in the second site in the chair conformation in a similar way to which it binds to hemagglutinin. The residues that interact with Neu5Ac at this second site are mostly conserved in avian strains, but not in human and swine strains, indicating that it has some as-yet-unknown biological function in birds.
Figures
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

