Disruption of syntaxin-mediated protein interactions blocks neurotransmitter secretion
- PMID: 9342384
- PMCID: PMC23745
- DOI: 10.1073/pnas.94.22.12186
Disruption of syntaxin-mediated protein interactions blocks neurotransmitter secretion
Abstract
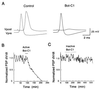
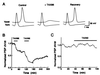
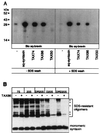
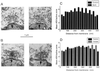
The membrane protein syntaxin participates in several protein-protein interactions that have been implicated in neurotransmitter release. To probe the physiological importance of these interactions, we microinjected into the squid giant presynaptic terminal botulinum toxin C1, which cleaves syntaxin, and the H3 domain of syntaxin, which mediates binding to other proteins. Both reagents inhibited synaptic transmission yet did not affect the number or distribution of synaptic vesicles at the presynaptic active zone. Recombinant H3 domain inhibited the interactions between syntaxin and SNAP-25 that underlie the formation of stable SNARE complexes in vitro. These data support the notion that syntaxin-mediated SNARE complexes are necessary for docked synaptic vesicles to fuse.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

