Inhibition of carbamyl phosphate synthetase-I and glutamine synthetase by hepatotoxic doses of acetaminophen in mice
- PMID: 9344900
- PMCID: PMC5127704
- DOI: 10.1006/taap.1997.8228
Inhibition of carbamyl phosphate synthetase-I and glutamine synthetase by hepatotoxic doses of acetaminophen in mice
Abstract
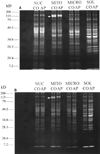
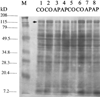
The primary mechanisms proposed for acetaminophen-induced hepatic necrosis should deplete protein thiols, either by covalent binding and thioether formation or by oxidative reactions such as S-thiolations. However, in previous studies we did not detect significant losses of protein thiol contents in response to administration of hepatotoxic doses of acetaminophen in vivo. In the present study we employed derivatization with the thiol-specific agent monobromobimane and separation of proteins by SDS-PAGE to investigate the possible loss of specific protein thiols during the course of acetaminophen-induced hepatic necrosis. Fasted adult male mice were given acetaminophen, and protein thiol status was examined subsequently in subcellular fractions isolated by differential centrifugation. No decreases in protein thiol contents were indicated, with the exception of a marked decrease in the fluorescent intensity, but not of protein content, as indicated by staining with Coomassie blue, of a single band of approximately 130 kDa in the mitochondrial fractions of acetaminophen-treated mice. This protein was identified by isolation and N-terminal sequence analysis as carbamyl phosphate synthetase-I (CPS-I) (EC 6.3.4.16). Hepatic CPS-I activities were decreased in mice given hepatotoxic doses of acetaminophen. In addition, hepatic glutamine synthetase activities were lower, and plasma ammonia levels were elevated in mice given hepatotoxic doses of acetaminophen. The observed hyperammonemia may contribute to the adverse effects of toxic doses of acetaminophen, and elucidation of the specific mechanisms responsible for the hyperammonemia may prove to be useful clinically. However, the preferential depletion of protein thiol content of a mitochondrial protein by chemically reactive metabolites generated in the endoplasmic reticulum presents a challenging and potentially informative mechanistic question.
Copyright 1997 Academic Press.
Figures









Similar articles
-
Acetaminophen-induced suppression of hepatic AdoMet synthetase activity is attenuated by prodrugs of L-cysteine.Toxicol Lett. 2002 Jun 7;132(1):1-8. doi: 10.1016/s0378-4274(01)00549-5. Toxicol Lett. 2002. PMID: 12084614
-
Role of CYP1A2 in the hepatotoxicity of acetaminophen: investigations using Cyp1a2 null mice.Toxicol Appl Pharmacol. 1998 Nov;153(1):102-8. doi: 10.1006/taap.1998.8543. Toxicol Appl Pharmacol. 1998. PMID: 9875304
-
Protective effects of garlic and related organosulfur compounds on acetaminophen-induced hepatotoxicity in mice.Toxicol Appl Pharmacol. 1996 Jan;136(1):146-54. doi: 10.1006/taap.1996.0018. Toxicol Appl Pharmacol. 1996. PMID: 8560468
-
Carbamyl phosphate synthetase III, an evolutionary intermediate in the transition between glutamine-dependent and ammonia-dependent carbamyl phosphate synthetases.J Mol Biol. 1994 Oct 14;243(1):131-40. doi: 10.1006/jmbi.1994.1638. J Mol Biol. 1994. PMID: 7932737 Review.
-
Mechanism and regulation of the glutamine-dependent carbamyl phosphate synthetase of Escherichia coli.Adv Enzymol Relat Areas Mol Biol. 1989;62:315-74. doi: 10.1002/9780470123089.ch7. Adv Enzymol Relat Areas Mol Biol. 1989. PMID: 2658488 Review. No abstract available.
Cited by
-
System Biology Investigation Revealed Lipopolysaccharide and Alcohol-Induced Hepatocellular Carcinoma Resembled Hepatitis B Virus Immunobiology and Pathogenesis.Int J Mol Sci. 2023 Jul 6;24(13):11146. doi: 10.3390/ijms241311146. Int J Mol Sci. 2023. PMID: 37446321 Free PMC article.
-
Carnitine and/or Acetylcarnitine Deficiency as a Cause of Higher Levels of Ammonia.Biomed Res Int. 2016;2016:2920108. doi: 10.1155/2016/2920108. Epub 2016 Feb 21. Biomed Res Int. 2016. PMID: 26998483 Free PMC article. Clinical Trial.
-
Mycoplasma pneumoniae infection and environmental tobacco smoke inhibit lung glutathione adaptive responses and increase oxidative stress.Infect Immun. 2008 Oct;76(10):4455-62. doi: 10.1128/IAI.00136-08. Epub 2008 Jul 21. Infect Immun. 2008. PMID: 18644874 Free PMC article.
-
Unveiling the molecular basis of paracetamol-induced hepatotoxicity: Interaction of N-acetyl-p-benzoquinone imine with mitochondrial succinate dehydrogenase.Biochem Biophys Rep. 2024 May 7;38:101727. doi: 10.1016/j.bbrep.2024.101727. eCollection 2024 Jul. Biochem Biophys Rep. 2024. PMID: 38766381 Free PMC article.
-
l-Alanine activates hepatic AMP-activated protein kinase and modulates systemic glucose metabolism.Mol Metab. 2018 Nov;17:61-70. doi: 10.1016/j.molmet.2018.08.002. Epub 2018 Aug 11. Mol Metab. 2018. PMID: 30190193 Free PMC article.
References
-
- Bartolone JB, Birge RB, Sparks K, Cohen SD, Khairallah EA. Immunochemical analysis of acetaminophen covalent binding to proteins. Partial characterization of the major acetaminophen-binding liver proteins. Biochem. Pharmacol. 1988;37:4763–4774. - PubMed
-
- Birge RB, Bartolone JB, Nishanian EV, Bruno MK, Mangold JB, Cohen SD, Khairallah EA. Dissociation of covalent binding from the oxidative effects of acetaminophen. Studies using dimethylated acetaminophen derivatives. Biochem. Pharmacol. 1988;37:3383–3393. - PubMed
-
- Birge RB, Bartolone JB, Cohen SD, Khairallah EA, Smolin LA. A comparison of proteins S-thiolated by glutathione to those arylated by acetaminophen. Biochem. Pharmacol. 1991;42:S197–S207. - PubMed
-
- Black M. Acetaminophen hepatotoxicity. Annu. Rev. Med. 1984;35:577–593. - PubMed
-
- Bodell WJ, Aida T, Berger MS, Rosenblum ML. Increased repair of O6-alkylguanine DNA adducts in glioma-derived human cells resistant to the cytotoxic and cytogenetic effects of 1,3-bis(2-chloroethyl)-1-nitrosourea. Carcinogenesis. 1986;7:879–883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

