Brush border myosin-I structure and ADP-dependent conformational changes revealed by cryoelectron microscopy and image analysis
- PMID: 9348285
- PMCID: PMC2141714
- DOI: 10.1083/jcb.139.3.683
Brush border myosin-I structure and ADP-dependent conformational changes revealed by cryoelectron microscopy and image analysis
Abstract
Brush border myosin-I (BBM-I) is a single-headed myosin found in the microvilli of intestinal epithelial cells, where it forms lateral bridges connecting the core bundle of actin filaments to the plasma membrane. Extending previous observations (Jontes, J.D., E.M. Wilson-Kubalek, and R.A. Milligan. 1995. Nature [Lond.]. 378:751-753), we have used cryoelectron microscopy and helical image analysis to generate three-dimensional (3D) maps of actin filaments decorated with BBM-I in both the presence and absence of 1 mM MgADP. In the improved 3D maps, we are able to see the entire light chain-binding domain, containing density for all three calmodulin light chains. This has enabled us to model a high resolution structure of BBM-I using the crystal structures of the chicken skeletal muscle myosin catalytic domain and essential light chain. Thus, we are able to directly measure the full magnitude of the ADP-dependent tail swing. The approximately 31 degrees swing corresponds to approximately 63 A at the end of the rigid light chain-binding domain. Comparison of the behavior of BBM-I with skeletal and smooth muscle subfragments-1 suggests that there are substantial differences in the structure and energetics of the biochemical transitions in the actomyosin ATPase cycle.
Figures

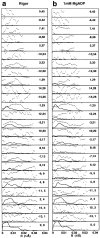

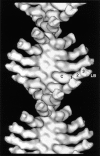

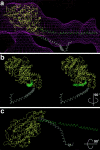
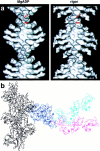

References
-
- Carragher BO, Whittaker M, Milligan RA. Helical processing using PHOELIX. J Struct Biol. 1996;116:107–112. - PubMed
-
- Cheney RE, Mooseker MS. Unconventional myosins. Curr Opin Cell Biol. 1992;4:27–35. - PubMed
-
- Collins JH, Borysenko CW. The 110,000-dalton actin- and calmodulin-binding protein from intestinal brush border is a myosin-like ATPase. J Biol Chem. 1984;259:14128–14135. - PubMed

