Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes
- PMID: 9348306
- PMCID: PMC2199122
- DOI: 10.1084/jem.186.9.1487
Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes
Abstract
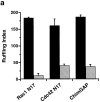
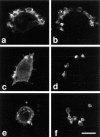
Specific pathways linking heterotrimeric G proteins and Fcgamma receptors to the actin-based cytoskeleton are poorly understood. To test a requirement for Rho family members in cytoskeletal events mediated by structurally diverse receptors in leukocytes, we transfected the full-length human chemotactic peptide receptor in RAW 264.7 cells and examined cytoskeletal alterations in response to the chemotactic peptide formyl-methionyl-leucyl-phenylalanine (FMLP), colony stimulating factor-1 (CSF-1), IgG-coated particles, and phorbol 12-myristate 13-acetate (PMA). Expression of Rac1 N17, Cdc42 N17, or the GAP domain of n-chimaerin inhibited cytoskeletal responses to FMLP and CSF-1, and blocked phagocytosis. Accumulation of F-actin- rich "phagocytic cups" was partially inhibited by expression of Rac1 N17 or Cdc42 N17. In contrast, PMA-induced ruffling was not inhibited by expression of Rac1 N17, but was blocked by expression of Cdc42 N17, indicating that cytoskeletal inhibition by these constructs was nonoverlapping. These results demonstrate differential requirements for Rho family GTPases in leukocyte motility, and indicate that both Rac1 and Cdc42 are required for Fcgamma receptor- mediated phagocytosis and for membrane ruffling mediated by structurally distinct receptors in macrophages.
Figures











Similar articles
-
Localization of p21-activated kinase 1 (PAK1) to pseudopodia, membrane ruffles, and phagocytic cups in activated human neutrophils.J Leukoc Biol. 1999 Sep;66(3):521-7. doi: 10.1002/jlb.66.3.521. J Leukoc Biol. 1999. PMID: 10496324
-
ARF6 is required for growth factor- and rac-mediated membrane ruffling in macrophages at a stage distal to rac membrane targeting.Mol Cell Biol. 1999 Dec;19(12):8158-68. doi: 10.1128/MCB.19.12.8158. Mol Cell Biol. 1999. PMID: 10567541 Free PMC article.
-
The GTPase-activating protein n-chimaerin cooperates with Rac1 and Cdc42Hs to induce the formation of lamellipodia and filopodia.Mol Cell Biol. 1996 Sep;16(9):5069-80. doi: 10.1128/MCB.16.9.5069. Mol Cell Biol. 1996. PMID: 8756665 Free PMC article.
-
Ras-related GTPases and the cytoskeleton.Mol Biol Cell. 1992 May;3(5):475-9. doi: 10.1091/mbc.3.5.475. Mol Biol Cell. 1992. PMID: 1611153 Free PMC article. Review.
-
Neutrophil chemoattractant receptors and the membrane skeleton.Bioessays. 1994 Mar;16(3):193-8. doi: 10.1002/bies.950160310. Bioessays. 1994. PMID: 8166673 Review.
Cited by
-
Rac1 modulates acute and subacute genotoxin-induced hepatic stress responses, fibrosis and liver aging.Cell Death Dis. 2013 Mar 21;4(3):e558. doi: 10.1038/cddis.2013.57. Cell Death Dis. 2013. PMID: 23519127 Free PMC article.
-
Essential diurnal Rac1 activation during retinal phagocytosis requires αvβ5 integrin but not tyrosine kinases focal adhesion kinase or Mer tyrosine kinase.Mol Biol Cell. 2012 Mar;23(6):1104-14. doi: 10.1091/mbc.E11-10-0840. Epub 2012 Jan 19. Mol Biol Cell. 2012. PMID: 22262456 Free PMC article.
-
The chemotactic defect in wiskott-Aldrich syndrome macrophages is due to the reduced persistence of directional protrusions.PLoS One. 2012;7(1):e30033. doi: 10.1371/journal.pone.0030033. Epub 2012 Jan 18. PLoS One. 2012. PMID: 22279563 Free PMC article.
-
Leukotriene B4 augments and restores Fc gammaRs-dependent phagocytosis in macrophages.J Biol Chem. 2010 Dec 24;285(52):41113-21. doi: 10.1074/jbc.M110.175497. Epub 2010 Oct 19. J Biol Chem. 2010. PMID: 20959460 Free PMC article.
-
A role for PKC-epsilon in Fc gammaR-mediated phagocytosis by RAW 264.7 cells.J Cell Biol. 2002 Dec 23;159(6):939-44. doi: 10.1083/jcb.200205140. Epub 2002 Dec 23. J Cell Biol. 2002. PMID: 12499353 Free PMC article.
References
-
- Tapon N, Hall A. Rho, Rac, and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. - PubMed
-
- Lim L, Hall C, Monfries C. Regulation of actin cytoskeleton by Rho-family GTPases and their associated proteins. Sem Cell Dev Biol. 1996;7:699–706.
-
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor–induced membrane ruffling. Cell. 1992;70:401–410. - PubMed
-
- Allen WE, Jones GE, Pollard JW, Ridley AJ. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci. 1997;110:707–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

