Specific activation of the cysteine protease CPP32 during the negative selection of T cells in the thymus
- PMID: 9348308
- PMCID: PMC2199117
- DOI: 10.1084/jem.186.9.1503
Specific activation of the cysteine protease CPP32 during the negative selection of T cells in the thymus
Abstract
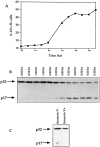
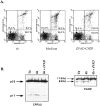
Cysteine proteases of the CED-3 and ICE family have been recently proposed as the ultimate executioners in several mammalian cell death pathways. Among them, the cysteine protease CPP32 has been shown to participate in programmed cell death (PCD), or apoptosis, affecting lymphoid cells in vitro. In the thymus, negative selection is a mechanism through which developing thymocytes expressing a TcR with high affinity for self peptide-MHC complexes are eliminated by PCD. In order to investigate the role of CPP32 in thymic apoptosis, isolated thymocytes were submitted to cell surface CD3 crosslinking by immobilized anti-CD3 mAb or to dexamethasone treatment. Although apoptosis occurred in the absence or after crosslinking with anti-CD3 mAb, specific activation of CPP32, as assessed by the extent of proteolytic cleavage of the p32 zymogen, was only detected in thymocytes cultured in the presence of the immobilized antibody or dexamethasone. This activation was a very early event during apoptosis as it occurred before the exposure of phosphatidyl serine to the upper side of the cell membrane. This was observed both in anti-CD3- and dexamethasone-induced apoptosis. Moreover, using mice transgenic for pigeon cytochrome C (PCC)-specific TcR, we were able to show that, after injection of PCC, the activation of CPP32 and cleavage of its substrate occurred in thymocytes obtained from mice expressing a permissive MHC haplotype for PCC presentation (H-2k). Moreover, PCC induced apoptosis was blocked by the caspase inhibitor zVAD. While spontaneous apoptosis was not accompanied by detectable levels of CPP32 processing, it was characterized by the proteolysis of poly(ADP-ribose) polymerase (PARP) and was blocked by the cysteine protease inhibitor, zVAD-CH2F. Taken together, these results support the concept that CPP32 is among the earliest effectors of the pathway leading to negative selection of autoreactive thymocytes. Our results also suggest the involvement of a distinct CPP32-like cysteine protease in spontaneous apoptosis of thymocytes.
Figures
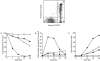


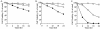



References
-
- Steller H. Mechanisms and genes of cellular suicide. Science (Wash DC) 1995;267:1445–1449. - PubMed
-
- Martin SJ, Green DR. Protease activation during apoptosis: death by a thousand cuts? . Cell. 1995;82:349–352. - PubMed
-
- Fraser A, Evan G. A license to kill. Cell. 1996;85:781–784. - PubMed
-
- Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis eleganscell death protein Ced-3 and mammalian interleukin-1 β-converting enzyme. J Biol Chem. 1994;269:30761–30764. - PubMed
-
- Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zheng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. YAMA/CPP32β, a mammalian homolog of CED-3, is a Crma-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

