Enforced Bcl-2 expression inhibits antigen-mediated clonal elimination of peripheral B cells in an antigen dose-dependent manner and promotes receptor editing in autoreactive, immature B cells
- PMID: 9348309
- PMCID: PMC2199120
- DOI: 10.1084/jem.186.9.1513
Enforced Bcl-2 expression inhibits antigen-mediated clonal elimination of peripheral B cells in an antigen dose-dependent manner and promotes receptor editing in autoreactive, immature B cells
Abstract
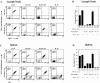
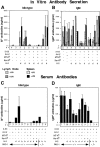
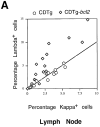
The mechanisms that establish immune tolerance in immature and mature B cells appear to be distinct. Membrane-bound autoantigen is thought to induce developmental arrest and receptor editing in immature B cells, whereas mature B cells have shortened lifespans when exposed to the same stimulus. In this study, we used Emu-bcl-2-22 transgenic (Tg) mice to test the prediction that enforced expression of the Bcl-2 apoptotic inhibitor in B cells would rescue mature, but not immature, B cells from tolerance induction. To monitor tolerance to the natural membrane autoantigen H-2Kb, we bred 3-83mudelta (anti-Kk,b) Ig Tg mice to H-2(b) mice or to mice expressing transgene-driven Kb in the periphery. In 3-83mudelta/bcl-2 Tg mice, deletion of autoreactive B cells induced by peripheral Kb antigen expression in the liver (MT-Kb Tg) or epithelia (KerIV-Kb Tg), was partly or completely inhibited, respectively. Furthermore, Bcl-2 protected peritoneal B-2 B cells from deletion mediated by acute antigen exposure, but this protection could be overcome by higher antigen dose. In contrast to its ability to block peripheral self-tolerance, Bcl-2 overexpression failed to inhibit central tolerance induced by bone marrow antigen expression, but instead, enhanced the receptor editing process. These studies indicate that apoptosis plays distinct roles in central and peripheral B cell tolerance.
Figures






References
-
- Cory S. Regulation of lymphocyte survival by the bcl-2 gene family. Annu Rev Immunol. 1995;13:513–543. - PubMed
-
- Green DR, Scott DW. Activation-induced apoptosis in lymphocytes. Curr Opin Immunol. 1994;3:476–487. - PubMed
-
- Krammer PH, Behrmann I, Daniel P, Dhein J, Debatin KM. Regulation of apoptosis in the immune system. Curr Opin Immunol. 1994;2:279–289. - PubMed
-
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science (Wash DC) 1995;5203:1456–1462. - PubMed
-
- Nossal GJ. Negative selection of lymphocytes. Cell. 1994;2:229–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

