Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production
- PMID: 9348319
- PMCID: PMC2199106
- DOI: 10.1084/jem.186.9.1603
Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production
Abstract
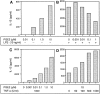
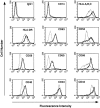
Interleukin (IL)-12 is a proinflammatory cytokine that contributes to innate resistance and to the development of antigen-specific T cell responses. Among other effects, prostaglandin E2 (PGE2) inhibits the production of IL-12 by macrophages activated with lipopolysaccharide (LPS). Here we investigated the effects of PGE2 on human dendritic cells (DCs) which develop in the presence of GM-CSF and IL-4. We demonstrate that in the absence of LPS, PGE2 dose dependently stimulated the production of IL-12 by DCs. Although PGE2 alone stimulated the production of low amounts of IL-12 only, it synergized with tumor necrosis factor (TNF)-alpha to induce high levels of IL-12 production by DCs. Addition of TNF-alpha in the absence of PGE2 had no effect on IL-12 production. Conversely, in the presence of LPS, PGE2 inhibited IL-12 production by DCs in a dose-dependent manner. The combination of PGE2 and TNF-alpha efficiently silenced mannose receptor-mediated endocytosis in DCs and readily induced neo-expression of the CD83 antigen. In addition, the expression of various surface antigens such as major histocompatibility complex class I and II, adhesion, as well as costimulatory molecules was upregulated by this treatment. The effects of PGE2 on IL-12 synthesis and CD83 expression could be mimicked by dibutyryl-cAMP and forskolin, indicating that they were due to the intracellular elevation of cAMP levels. DC treated with PGE2 and TNF-alpha were most potent in stimulating allogeneic T cell proliferation. Our data demonstrate that PGE2 contributes to the maturation of human DCs and that PGE2 can be a potent enhancer of IL-12 production by human DCs.
Figures



References
-
- Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science (Wash DC) 1996;272:50–53. - PubMed
-
- Garrison, J.C. 1990. Histamin, bradykinin, 5-hydroxytryptamine and their antagonists. In The Pharmacological Basis of Therapeutics. A. Gilman, T.W. Rall, A.S. Nies, and P. Taylor, editors. Pergamon Press, New York. 574–599.
-
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. - PubMed
-
- Vane, J.R., and R.M. Botting. 1994. Biological properties of cyclooxygenase products. In The Handbook of Immunopharmacology. F.M. Cunningham, editor. Academic Press, London. 61–97.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

