Nociceptin inhibits T-type Ca2+ channel current in rat sensory neurons by a G-protein-independent mechanism
- PMID: 9348341
- PMCID: PMC6573070
- DOI: 10.1523/JNEUROSCI.17-22-08721.1997
Nociceptin inhibits T-type Ca2+ channel current in rat sensory neurons by a G-protein-independent mechanism
Abstract
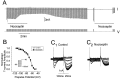
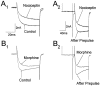
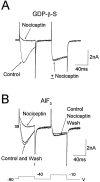
Nociceptin (orphanin FQ) is a novel, opioid-like, heptadecapeptide that is an endogenous ligand for the opioid receptor-like (ORL1) receptor. Unlike classical opioids, nociceptin can produce hyperalgesia when injected intracerebroventricularly into mice. Despite this, nociceptin has been reported to decrease transmitter release, activate an inwardly rectifying K+ conductance, and suppress high-voltage-activated Ca2+ channel conductances (HVA gCa) in much the same way as micro-, delta-, and kappa-opioids. We report an action of nociceptin that is not shared by morphine: the suppression of low-voltage-activated, transient calcium (barium) current (IBa,T) in acutely dissociated rat dorsal root ganglion (DRG) neurons (EC50 = 100 nM). This effect was reflected as inhibition of bursts of action potentials that can be evoked in "medium-sized" DRG neurons. Experiments with GTP-gamma-S (100 microM), GDP-beta-S (2 mM), or aluminum fluoride (AlF3) (100 microM) in the patch pipette failed to provide evidence for G-protein involvement in nociceptin-induced IBa,T suppression. By contrast, both morphine and nociceptin suppressed HVA gCa, and the latter response was affected by intracellular GTP-gamma-S, GDP-beta-S, and AlF3 in ways that confirmed G-protein involvement. The selective effect of nociceptin on IBa,T may therefore be relevant to understanding why its behavioral actions differ from those of other opioids. This G-protein-independent effect of the action of nociceptin may reflect a new general mechanism of action for opioid peptides within the nervous system.
Figures




References
-
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. - PubMed
-
- Brauneis U, Oz M, Peoples RW, Weight FF, Zhang I. Differential sensitivity of recombinant N-methyl d-aspartate receptor subunits to inhibition by dynorphin. J Pharmacol Exp Ther. 1996;279:1063–1068. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
