14-3-3 inhibits the Dictyostelium myosin II heavy-chain-specific protein kinase C activity by a direct interaction: identification of the 14-3-3 binding domain
- PMID: 9348531
- PMCID: PMC25635
- DOI: 10.1091/mbc.8.10.1889
14-3-3 inhibits the Dictyostelium myosin II heavy-chain-specific protein kinase C activity by a direct interaction: identification of the 14-3-3 binding domain
Abstract
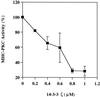
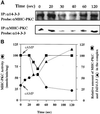
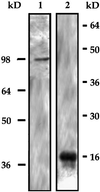
Myosin II heavy chain (MHC) specific protein kinase C (MHC-PKC), isolated from Dictyostelium discoideum, regulates myosin II assembly and localization in response to the chemoattractant cyclic AMP. Immunoprecipitation of MHC-PKC revealed that it resides as a complex with several proteins. We show herein that one of these proteins is a homologue of the 14-3-3 protein (Dd14-3-3). This protein has recently been implicated in the regulation of intracellular signaling pathways via its interaction with several signaling proteins, such as PKC and Raf-1 kinase. We demonstrate that the mammalian 14-3-3 zeta isoform inhibits the MHC-PKC activity in vitro and that this inhibition is carried out by a direct interaction between the two proteins. Furthermore, we found that the cytosolic MHC-PKC, which is inactive, formed a complex with Dd14-3-3 in the cytosol in a cyclic AMP-dependent manner, whereas the membrane-bound active MHC-PKC was not found in a complex with Dd14-3-3. This suggests that Dd14-3-3 inhibits the MHC-PKC in vivo. We further show that MHC-PKC binds Dd14-3-3 as well as 14-3-3 zeta through its C1 domain, and the interaction between these two proteins does not involve a peptide containing phosphoserine as was found for Raf-1 kinase. Our experiments thus show an in vivo function for a member of the 14-3-3 family and demonstrate that MHC-PKC interacts directly with Dd14-3-3 and 14-3-3 zeta through its C1 domain both in vitro and in vivo, resulting in the inhibition of the kinase.
Figures

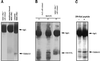


Similar articles
-
Chemoattractant-mediated increases in cGMP induce changes in Dictyostelium myosin II heavy chain-specific protein kinase C activities.J Cell Biol. 1996 Aug;134(4):911-21. doi: 10.1083/jcb.134.4.911. J Cell Biol. 1996. PMID: 8769416 Free PMC article.
-
Autophosphorylation of Dictyostelium myosin II heavy chain-specific protein kinase C is required for its activation and membrane dissociation.J Biol Chem. 1997 Jan 10;272(2):828-34. doi: 10.1074/jbc.272.2.828. J Biol Chem. 1997. PMID: 8995370
-
Dictyostelium myosin II is regulated during chemotaxis by a novel protein kinase C.J Biol Chem. 1996 Jan 12;271(2):977-84. doi: 10.1074/jbc.271.2.977. J Biol Chem. 1996. PMID: 8557714
-
Insight into intra- and inter-molecular interactions of PKC: design of specific modulators of kinase function.Pharmacol Res. 2007 Jun;55(6):467-76. doi: 10.1016/j.phrs.2007.04.014. Epub 2007 May 3. Pharmacol Res. 2007. PMID: 17580120 Free PMC article. Review.
-
14-3-3 proteins: biological function and domain structure.Biochem Soc Trans. 1995 Aug;23(3):605-11. doi: 10.1042/bst0230605. Biochem Soc Trans. 1995. PMID: 8566426 Review. No abstract available.
Cited by
-
A 20-amino acid module of protein kinase C{epsilon} involved in translocation and selective targeting at cell-cell contacts.J Biol Chem. 2009 Jul 10;284(28):18808-15. doi: 10.1074/jbc.M109.004614. Epub 2009 May 8. J Biol Chem. 2009. PMID: 19429675 Free PMC article.
-
The yeast 14-3-3 proteins BMH1 and BMH2 differentially regulate rapamycin-mediated transcription.Biosci Rep. 2014 Apr 1;34(2):e00099. doi: 10.1042/BSR20130096. Print 2014 Apr 1. Biosci Rep. 2014. PMID: 27919033 Free PMC article.
-
Protein kinase Cepsilon actin-binding site is important for neurite outgrowth during neuronal differentiation.Mol Biol Cell. 2002 Jan;13(1):12-24. doi: 10.1091/mbc.01-04-0210. Mol Biol Cell. 2002. PMID: 11809819 Free PMC article.
-
Specific interactions with TBP and TFIIB in vitro suggest that 14-3-3 proteins may participate in the regulation of transcription when part of a DNA binding complex.Plant Cell. 1999 Aug;11(8):1591-602. doi: 10.1105/tpc.11.8.1591. Plant Cell. 1999. PMID: 10449590 Free PMC article.
-
Protein kinase C activation by acidic proteins including 14-3-3.Biochem J. 2000 May 1;347 Pt 3(Pt 3):781-5. doi: 10.1042/0264-6021:3470781. Biochem J. 2000. PMID: 10769183 Free PMC article.
References
-
- Abu-Elneel K, Karchi M, Ravid S. Dictyostelium myosin II is regulated during chemotaxis by a novel protein kinase C. J Biol Chem. 1996;271:977–984. - PubMed
-
- Aitken A. 14–3-3 proteins on the MAP. Trends Biochem Sci. 1995;20:95–97. - PubMed
-
- Aitken A. 14–3-3 and its possible role in coordinating multiple signalling pathways. Trends Cell Biol. 1996;6:341–347. - PubMed
-
- Aitken A, Ellis CA, Harris A, Sellers LA, Toker A. Kinase and neurotransmitters. Nature. 1990;344:594–596. - PubMed
-
- Aitken A, Howell S, Jones D, Madrazo J, Martin H, Patel Y, Robinson K. Post-translationally modified 14–3-3 isoforms and inhibition of protein kinase C. Mol Cell Biochem. 1995a;149/150:41–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

