Segregation of two spectrin isoforms: polarized membrane-binding sites direct polarized membrane skeleton assembly
- PMID: 9348534
- PMCID: PMC25644
- DOI: 10.1091/mbc.8.10.1933
Segregation of two spectrin isoforms: polarized membrane-binding sites direct polarized membrane skeleton assembly
Abstract
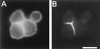
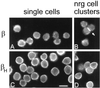
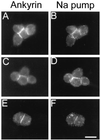
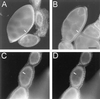
Spectrin isoforms are often segregated within specialized plasma membrane subdomains where they are thought to contribute to the development of cell surface polarity. It was previously shown that ankyrin and beta spectrin are recruited to sites of cell-cell contact in Drosophila S2 cells expressing the homophilic adhesion molecule neuroglian. Here, we show that neuroglian has no apparent effect on a second spectrin isoform (alpha beta H), which is constitutively associated with the plasma membrane in S2 cells. Another membrane marker, the Na,K-ATPase, codistributes with ankyrin and alpha beta spectrin at sites of neuroglian-mediated contact. The distributions of these markers in epithelial cells in vivo are consistent with the order of events observed in S2 cells. Neuroglian, ankyrin, alpha beta spectrin, and the Na,K-ATPase colocalize at the lateral domain of salivary gland cells. In contrast, alpha beta H spectrin is sorted to the apical domain of salivary gland and somatic follicle cells. Thus, the two spectrin isoforms respond independently to positional cues at the cell surface: in one case an apically sorted receptor and in the other case a locally activated cell-cell adhesion molecule. The results support a model in which the membrane skeleton behaves as a transducer of positional information within cells.
Figures



Similar articles
-
Posterior midgut epithelial cells differ in their organization of the membrane skeleton from other drosophila epithelia.Exp Cell Res. 2001 Nov 1;270(2):176-87. doi: 10.1006/excr.2001.5343. Exp Cell Res. 2001. PMID: 11640882
-
Neuroglian-mediated cell adhesion induces assembly of the membrane skeleton at cell contact sites.J Cell Biol. 1996 May;133(3):647-55. doi: 10.1083/jcb.133.3.647. J Cell Biol. 1996. PMID: 8636238 Free PMC article.
-
Neuroglian and DE-cadherin activate independent cytoskeleton assembly pathways in Drosophila S2 cells.Biochem Biophys Res Commun. 1999 Nov 19;265(2):372-5. doi: 10.1006/bbrc.1999.1689. Biochem Biophys Res Commun. 1999. PMID: 10558874
-
An Adaptable Spectrin/Ankyrin-Based Mechanism for Long-Range Organization of Plasma Membranes in Vertebrate Tissues.Curr Top Membr. 2016;77:143-84. doi: 10.1016/bs.ctm.2015.10.001. Epub 2015 Nov 30. Curr Top Membr. 2016. PMID: 26781832 Review.
-
Role of the membrane-cytoskeleton in the spatial organization of the Na,K-ATPase in polarized epithelial cells.Soc Gen Physiol Ser. 1991;46:77-87. Soc Gen Physiol Ser. 1991. PMID: 1653995 Review.
Cited by
-
Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila.J Cell Biol. 2003 Jun 9;161(5):979-89. doi: 10.1083/jcb.200212054. Epub 2003 Jun 2. J Cell Biol. 2003. PMID: 12782686 Free PMC article.
-
Crumbs interacts with moesin and beta(Heavy)-spectrin in the apical membrane skeleton of Drosophila.J Cell Biol. 2002 Sep 2;158(5):941-51. doi: 10.1083/jcb.200203080. Epub 2002 Sep 3. J Cell Biol. 2002. PMID: 12213838 Free PMC article.
-
Alpha spectrin is essential for morphogenesis and body wall muscle formation in Caenorhabditis elegans.J Cell Biol. 2002 May 13;157(4):665-77. doi: 10.1083/jcb.200111051. Epub 2002 May 6. J Cell Biol. 2002. PMID: 11994313 Free PMC article.
-
Structural requirements for outside-in and inside-out signaling by Drosophila neuroglian, a member of the L1 family of cell adhesion molecules.J Cell Biol. 1998 Jul 13;142(1):251-61. doi: 10.1083/jcb.142.1.251. J Cell Biol. 1998. PMID: 9660878 Free PMC article.
-
Competition between myosin II and βH-spectrin regulates cytoskeletal tension.Elife. 2023 Jun 27;12:RP84918. doi: 10.7554/eLife.84918. Elife. 2023. PMID: 37367948 Free PMC article.
References
-
- Anderson MS, Kunkel LM. The molecular and biochemical basis of Duchenne muscular dystrophy. Trends Biol Sci. 1992;17:289–292. - PubMed
-
- Beck KA, Nelson WJ. Once there, making the decision to stay or leave. In: Nelson WJ, editor. Current Topics in Membranes. Vol. 43. San Diego: Academic Press; 1996. pp. 15–25.
-
- Bennett V. Ankyrins. J Biol Chem. 1992;267:8703–8706. - PubMed
-
- Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. - PubMed
-
- Bieber AJ, Snow PM, Hortsch M, Patel NH, Jacobs JR, Traquina ZR, Schilling J, Goodman CS. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell. 1989;59:447–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

