Amphiphysin heterodimers: potential role in clathrin-mediated endocytosis
- PMID: 9348539
- PMCID: PMC25662
- DOI: 10.1091/mbc.8.10.2003
Amphiphysin heterodimers: potential role in clathrin-mediated endocytosis
Abstract
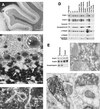
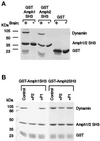
Amphiphysin (Amph) is a src homology 3 domain-containing protein that has been implicated in synaptic vesicle endocytosis as a result of its interaction with dynamin. In a screen for novel members of the amphiphysin family, we identified Amph2, an isoform 49% identical to the previously characterized Amph1 protein. The subcellular distribution of this isoform parallels Amph1, both being enriched in nerve terminals. Like Amph1, a role in endocytosis at the nerve terminal is supported by the rapid dephosphorylation of Amph2 on depolarization. Importantly, the two isoforms can be coimmunoprecipitated from the brain as an equimolar complex, suggesting that the two isoforms act in concert. As determined by cross-linking of brain extracts, the Amph1-Amph2 complex is a 220- to 250-kDa heterodimer. COS cells transfected with either Amph1 or Amph2 show greatly reduced transferrin uptake, but coexpression of the two proteins rescues this defect, supporting a role for the heterodimer in clathrin-mediated endocytosis. Although the src homology 3 domains of both isoforms interact with dynamin, the heterodimer can associate with multiple dynamin molecules in vitro and activates dynamin's GTPase activity. We propose that it is an amphiphysin heterodimer that drives the recruitment of dynamin to clathrin-coated pits in endocytosing nerve terminals.
Figures
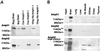



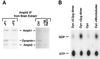

Similar articles
-
Inhibition of receptor-mediated endocytosis by the amphiphysin SH3 domain.Curr Biol. 1997 Aug 1;7(8):554-60. doi: 10.1016/s0960-9822(06)00254-5. Curr Biol. 1997. PMID: 9259551
-
Clathrin interacts specifically with amphiphysin and is displaced by dynamin.FEBS Lett. 1997 Aug 18;413(2):319-22. doi: 10.1016/s0014-5793(97)00928-9. FEBS Lett. 1997. PMID: 9280305
-
Importance of the pleckstrin homology domain of dynamin in clathrin-mediated endocytosis.Curr Biol. 1999 Mar 11;9(5):257-60. doi: 10.1016/s0960-9822(99)80114-6. Curr Biol. 1999. PMID: 10074456
-
Dynamin, endocytosis and intracellular signalling (review).Mol Membr Biol. 1996 Oct-Dec;13(4):189-215. doi: 10.3109/09687689609160598. Mol Membr Biol. 1996. PMID: 9116759 Review.
-
Amphiphysin I and regulation of synaptic vesicle endocytosis.Acta Med Okayama. 2009 Dec;63(6):305-23. doi: 10.18926/AMO/31822. Acta Med Okayama. 2009. PMID: 20035287 Review.
Cited by
-
Amphiphysin I but not dynamin I nor synaptojanin mRNA expression increased after repeated methamphetamine administration in the rat cerebrum and cerebellum.J Neural Transm (Vienna). 2013 Jul;120(7):1039-52. doi: 10.1007/s00702-012-0931-7. Epub 2012 Dec 8. J Neural Transm (Vienna). 2013. PMID: 23224692
-
Amphiphysin is necessary for organization of the excitation-contraction coupling machinery of muscles, but not for synaptic vesicle endocytosis in Drosophila.Genes Dev. 2001 Nov 15;15(22):2967-79. doi: 10.1101/gad.207801. Genes Dev. 2001. PMID: 11711432 Free PMC article.
-
Synaptophysin regulates clathrin-independent endocytosis of synaptic vesicles.Proc Natl Acad Sci U S A. 2000 May 23;97(11):6120-5. doi: 10.1073/pnas.97.11.6120. Proc Natl Acad Sci U S A. 2000. PMID: 10823955 Free PMC article.
-
Yeast as a Model to Understand Actin-Mediated Cellular Functions in Mammals-Illustrated with Four Actin Cytoskeleton Proteins.Cells. 2020 Mar 10;9(3):672. doi: 10.3390/cells9030672. Cells. 2020. PMID: 32164332 Free PMC article. Review.
-
Crystal structure of the amphiphysin-2 SH3 domain and its role in the prevention of dynamin ring formation.EMBO J. 1998 Sep 15;17(18):5273-85. doi: 10.1093/emboj/17.18.5273. EMBO J. 1998. PMID: 9736607 Free PMC article.
References
-
- Bauerfeind, R., David, C., and De Camilli, P. (1995). Amphiphysin, a nerve-terminal protein with a putative function in synaptic vesicle endocytosis, is dephosphorylated upon stimulation of neurotransmitter release. Mol. Biol. Cell 6 (suppl), 405a.
-
- Butler MH, David C, Ochoa GC, Freyberg Z, Daniell L, Grabs D, Cremona O, De Camilli P. Amphiphysin II (SH3P9; BIN1), a member of the amphiphysin/Rvs family, is concentrated in the cortical cytomatrix of axon initial segments and nodes of ranvier in brain and around T tubules in skeletal muscle. J Cell Biol. 1997;137:1355–1367. - PMC - PubMed
-
- Clark SG, Stern MJ, Horvitz HR. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992;356:340–344. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

