Cell-adhesive responses to tenascin-C splice variants involve formation of fascin microspikes
- PMID: 9348542
- PMCID: PMC25670
- DOI: 10.1091/mbc.8.10.2055
Cell-adhesive responses to tenascin-C splice variants involve formation of fascin microspikes
Abstract
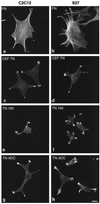
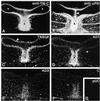
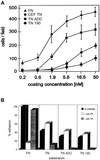
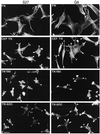
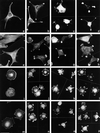
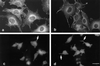
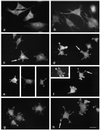
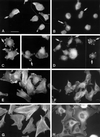
Tenascin-C is an adhesion-modulating matrix glycoprotein that has multiple effects on cell behavior. Tenascin-C transcripts are expressed in motile cells and at sites of tissue modeling during development, and alternative splicing generates variants that encode different numbers of fibronectin type III repeats. We have examined the in vivo expression and cell adhesive properties of two full-length recombinant tenascin-C proteins: TN-190, which contains the eight constant fibronectin type III repeats, and TN-ADC, which contains the additional AD2, AD1, and C repeats. In situ hybridization with probes specific for the AD2, AD1, and C repeats shows that these splice variants are expressed at sites of active tissue modeling and fibronectin expression in the developing avian feather bud and sternum. Transcripts incorporating the AD2, AD1, and C repeats are present in embryonic day 10 wing bud but not in embryonic day 10 lung. By using a panel of nine cell lines in attachment assays, we have found that C2C12, G8, and S27 myoblastic cells undergo concentration-dependent adhesion to both variants, organize actin microspikes that contain the actin-bundling protein fascin, and do not assemble focal contacts. On a molar basis, TN-ADC is more active than TN-190 in promoting cell attachment and irregular cell spreading. The addition of either TN-190 or TN-ADC in solution to C2C12, COS-7, or MG-63 cells adherent on fibronectin decreases cell attachment and results in decreased organization of actin microfilament bundles, with formation of cortical membrane ruffles and retention of residual points of substratum contact that contain filamentous actin and fascin. These data establish a biochemical similarity in the processes of cell adhesion to tenascin-C and thrombospondin-1, also an "antiadhesive" matrix component, and also demonstrate that both the adhesive and adhesion-modulating properties of tenascin-C involve similar biochemical events in the cortical cytoskeleton. In addition to these generic properties, TN-ADC is less active in adhesion modulation than TN-190. The coordinated expression of different tenascin-C transcripts during development may, therefore, provide appropriate microenvironments for regulated changes in cell shape, adhesion, and movement.
Figures



Similar articles
-
Cell-matrix adhesions differentially regulate fascin phosphorylation.Mol Biol Cell. 1999 Dec;10(12):4177-90. doi: 10.1091/mbc.10.12.4177. Mol Biol Cell. 1999. PMID: 10588651 Free PMC article.
-
Formation of stable microspikes containing actin and the 55 kDa actin bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1: implications for the anti-adhesive activities of thrombospondin-1.J Cell Sci. 1995 May;108 ( Pt 5):1977-90. doi: 10.1242/jcs.108.5.1977. J Cell Sci. 1995. PMID: 7657718
-
Characterization of cell-matrix adhesion requirements for the formation of fascin microspikes.Mol Biol Cell. 1997 Nov;8(11):2345-63. doi: 10.1091/mbc.8.11.2345. Mol Biol Cell. 1997. PMID: 9362073 Free PMC article.
-
Biologically Active TNIIIA2 Region in Tenascin-C Molecule: A Major Contributor to Elicit Aggressive Malignant Phenotypes From Tumors/Tumor Stroma.Front Immunol. 2020 Dec 9;11:610096. doi: 10.3389/fimmu.2020.610096. eCollection 2020. Front Immunol. 2020. PMID: 33362799 Free PMC article. Review.
-
The structure and function of tenascins in the nervous system.Matrix Biol. 2001 Feb;20(1):13-22. doi: 10.1016/s0945-053x(00)00136-0. Matrix Biol. 2001. PMID: 11246000 Review.
Cited by
-
Tenascin C in metastasis: A view from the invasive front.Cell Adh Migr. 2015;9(1-2):112-24. doi: 10.1080/19336918.2015.1008331. Cell Adh Migr. 2015. PMID: 25738825 Free PMC article. Review.
-
Cell-matrix adhesions differentially regulate fascin phosphorylation.Mol Biol Cell. 1999 Dec;10(12):4177-90. doi: 10.1091/mbc.10.12.4177. Mol Biol Cell. 1999. PMID: 10588651 Free PMC article.
-
Existence of tenascin-C isoforms in rat that contain the alternatively spliced AD1 domain are developmentally regulated during hippocampal development.Cell Mol Neurobiol. 2012 Mar;32(2):279-87. doi: 10.1007/s10571-011-9759-1. Epub 2011 Oct 4. Cell Mol Neurobiol. 2012. PMID: 21968644 Free PMC article.
-
Expressed miRNAs target feather related mRNAs involved in cell signaling, cell adhesion and structure during chicken epidermal development.Gene. 2016 Oct 15;591(2):393-402. doi: 10.1016/j.gene.2016.06.027. Epub 2016 Jun 15. Gene. 2016. PMID: 27320726 Free PMC article.
-
Independent prognostic value of fascin immunoreactivity in stage I nonsmall cell lung cancer.Br J Cancer. 2003 Feb 24;88(4):537-47. doi: 10.1038/sj.bjc.6600731. Br J Cancer. 2003. PMID: 12592367 Free PMC article.
References
-
- Adams JC. Formation of stable microspikes containing actin and the 55-kDa actin bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1: implications for the anti-adhesive activities of thrombospondin-1. J Cell Sci. 1995;108:1977–1990. - PubMed
-
- Adams JC, Lawler J. Diverse mechanisms for cell attachment to platelet thrombospondin. J Cell Sci. 1993;104:1061–1071. - PubMed
-
- Adams JC, Tucker RP, Lawler J. The Thrombospondin Gene Family. Austin, TX: R.G. Landes; 1995. and Berlin: Springer-Verlag.
-
- Asch AS, Tepler J, Silbiger S, Nachman RL. Cellular attachment to thrombospondin: cooperative interactions between receptor systems. J Biol Chem. 1991;266:1740–1745. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

