end-1 encodes an apparent GATA factor that specifies the endoderm precursor in Caenorhabditis elegans embryos
- PMID: 9353257
- PMCID: PMC316658
- DOI: 10.1101/gad.11.21.2883
end-1 encodes an apparent GATA factor that specifies the endoderm precursor in Caenorhabditis elegans embryos
Abstract
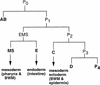
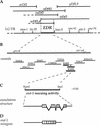
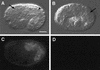
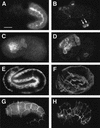
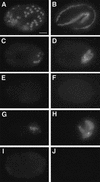
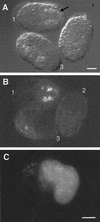
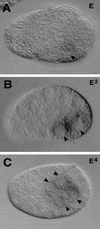
The endoderm in the nematode Caenorhabditis elegans is clonally derived from the E founder cell. We identified a single genomic region (the endoderm-determining region, or EDR) that is required for the production of the entire C. elegans endoderm. In embryos lacking the EDR, the E cell gives rise to ectoderm and mesoderm instead of endoderm and appears to adopt the fate of its cousin, the C founder cell. end-1, a gene from the EDR, restores endoderm production in EDR deficiency homozygotes. end-1 transcripts are first detectable specifically in the E cell, consistent with a direct role for end-1 in endoderm development. The END-1 protein is an apparent zinc finger-containing GATA transcription factor. As GATA factors have been implicated in endoderm development in other animals, our findings suggest that endoderm may be specified by molecularly conserved mechanisms in triploblastic animals. We propose that end-1, the first zygotic gene known to be involved in the specification of germ layer and founder cell identity in C. elegans, may link maternal genes that regulate the establishment of the endoderm to downstream genes responsible for endoderm differentiation.
Figures
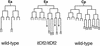
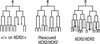


References
-
- Aamodt EJ, Chung MA, McGhee JD. Spatial control of gut-specific gene expression during Caenorhabditis elegans development. Science. 1991;252:579–582. - PubMed
-
- Albertson DG, Sulston JE, White JG. Cell cycling and DNA replication in a mutant blocked in cell division in the nematode Caenorhabditis elegans. Dev Biol. 1978;63:165–178. - PubMed
-
- Altschul SF, Gish W, Miller W, Myers W, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Austin J, Kimble J. Transcript analysis of glp-1 and lin-12, homologous genes required for cell interactions during development of C. elegans. Cell. 1989;58:565–571. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
